 | |
 | |
| Names | |
|---|---|
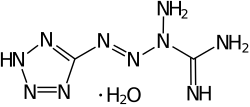
| IUPAC name 1-(5-tetrazolyl)-3-guanyl tetrazene hydrate | |
| Other names Tetracene [1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.128.336 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2H6N10·H2O | |
| Molar mass | 188.15 g/mol |
| Appearance | Pale yellow/colorless crystal plates [1] |
| Density | 1.7 g/cm3 |
| Boiling point | Decomposes at160 °C (320 °F; 433 K) |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
| Detonation velocity | ~4000 m/s |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tetrazene (1-(5-tetrazolyl)-3-guanyl tetrazene hydrate) [2] is an explosive material used for sensitization of priming compositions. It is a derivative of the compound with the IUPAC name tetrazene.
Contents
Tetrazene is slightly more impact-sensitive than mercury fulminate. When pressed enough, its sensitivity is reduced or destroyed; this is known as dead pressing. It also decomposes in boiling water. In contact with fire, it readily explodes, producing large amounts of black smoke. It is prepared by reacting sodium nitrite with an aminoguanidine salt dissolved in acetic acid at 30–40 °C.
Commercially, tetrazene is added in a small proportions to increase the sensitivity of lead styphnate in cap compositions used both in centre-fire (eg shotgun cartridges) and rim-fire (eg 0.22" ammunition) applications. Cap compositions also contain a high proportion of barium nitrate as an oxidising agent, and scintillating compounds such as antimony disulphide or ground glass which cause the heat of the explosion when struck by the firearm firing pin to rapidly dissipate the main charge of either nitrocellulose or cordite.