
Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.

The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria, archaea, and the mitochondria of eukaryotes.

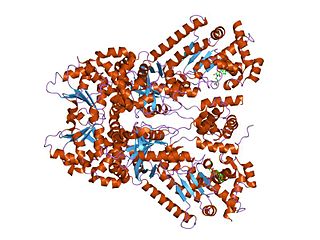
The coenzyme Q : cytochrome c – oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain, playing a critical role in biochemical generation of ATP. Complex III is a multisubunit transmembrane protein encoded by both the mitochondrial and the nuclear genomes. Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most bacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders. The bc1 complex contains 11 subunits, 3 respiratory subunits, 2 core proteins and 6 low-molecular weight proteins.

Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.

Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and oxidative phosphorylation. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential.

The membrane attack complex (MAC) or terminal complement complex (TCC) is a complex of proteins typically formed on the surface of pathogen cell membranes as a result of the activation of the host's complement system, and as such is an effector of the immune system. Antibody-mediated complement activation leads to MAC deposition on the surface of infected cells. Assembly of the MAC leads to pores that disrupt the cell membrane of target cells, leading to cell lysis and death.

In chemistry, an ionophore is a chemical species that reversibly binds ions. Many ionophores are lipid-soluble entities that transport ions across the cell membrane. Ionophores catalyze ion transport across hydrophobic membranes, such as liquid polymeric membranes or lipid bilayers found in the living cells or synthetic vesicles (liposomes). Structurally, an ionophore contains a hydrophilic center and a hydrophobic portion that interacts with the membrane.

Antimycin A is a secondary metabolite produced by Streptomyces bacteria and a member of a group of related compounds called antimycins. Antimycin A is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

Gp41 also known as glycoprotein 41 is a subunit of the envelope protein complex of retroviruses, including human immunodeficiency virus (HIV). Gp41 is a transmembrane protein that contains several sites within its ectodomain that are required for infection of host cells. As a result of its importance in host cell infection, it has also received much attention as a potential target for HIV vaccines.
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupt various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.
Cell-penetrating peptides (CPPs) are short peptides that facilitate cellular intake and uptake of molecules ranging from nanosize particles to small chemical compounds to large fragments of DNA. The "cargo" is associated with the peptides either through chemical linkage via covalent bonds or through non-covalent interactions.
Myxothiazol is a chemical compound produced by the myxobacterium Myxococcus fulvus. It is an inhibitor of the mitochondrial cytochrome bc1 complex.
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction
Metalloprotease inhibitors are cellular inhibitors of the Matrix metalloproteinases (MMPs). MMPs belong to a family of zinc-dependent neutral endopeptidases. These enzymes have the ability to break down connective tissue. The expression of MMPs is increased in various pathological conditions like inflammatory conditions, metabolic bone disease, to cancer invasion, metastasis and angiogenesis. Examples of diseases are periodontitis, hepatitis, glomerulonephritis, atherosclerosis, emphysema, asthma, autoimmune disorders of skin and dermal photoaging, rheumatoid arthritis, osteoarthritis, multiple sclerosis, Alzheimer's disease, chronic ulcerations, uterine involution, corneal epithelial defects, bone resorption and tumor progression and metastasis. Due to the role of MMPs in pathological conditions, inhibitors of MMPs may have therapeutic potential. Several other proteins have similar inhibitory effects, however none as effective. They might have other biological activities which have yet been fully characterised.

A channel blocker is the biological mechanism in which a particular molecule is used to prevent the opening of ion channels in order to produce a physiological response in a cell. Channel blocking is conducted by different types of molecules, such as cations, anions, amino acids, and other chemicals. These blockers act as ion channel antagonists, preventing the response that is normally provided by the opening of the channel.
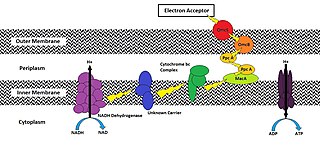
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.
The first human immunodeficiency virus (HIV) case was reported in the United States in the early 1980s. Many drugs have been discovered to treat the disease but mutations in the virus and resistance to the drugs make development difficult. Integrase is a viral enzyme that integrates retroviral DNA into the host cell genome. Integrase inhibitors are a new class of drugs used in the treatment of HIV. The first integrase inhibitor, raltegravir, was approved in 2007 and other drugs were in clinical trials in 2011.
Fish acute toxicity syndrome (FATS) is a set of common chemical and functional responses in fish resulting from a short-term, acute exposure to a lethal concentration of a toxicant, a chemical or material that can produce an unfavorable effect in a living organism. By definition, modes of action are characterized by FATS because the combination of common responses that represent each fish acute toxicity syndrome characterize an adverse biological effect. Therefore, toxicants that have the same mode of action elicit similar sets of responses in the organism and can be classified by the same fish acute toxicity syndrome.
Chelated platinum is an ionized form of platinum that forms two or more bonds with a counter ion. Some platinum chelates are claimed to have antimicrobial activity.














