A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Thiazole, or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Pyrazole is an organic compound of azole group with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.

Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine. Although benzoxazole itself is of little practical value, many derivatives of benzoxazoles are commercially important.
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.

In organic chemistry, episulfides are a class of organic compounds that contain a saturated, heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes. Episulfides are less common and generally less stable than epoxides. The most common derivative is ethylene sulfide.

Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound with the formula C2H4S. It is the smallest sulfur-containing heterocycle and the simplest episulfide. Like many organosulfur compounds, this species has a highly unpleasant odour. Thiirane is also used to describe any derivative of the parent ethylene sulfide.

Dibenzothiophene (DBT, diphenylene sulfide) is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It has the chemical formula C12H8S. It is a colourless solid that is chemically somewhat similar to anthracene. This tricyclic heterocycle, and especially its disubstituted derivative 4,6-dimethyldibenzothiophene are problematic impurities in petroleum.

1,3,5-Trithiane is the chemical compound with the formula (CH2S)3. This heterocycle is the cyclic trimer of the otherwise unstable species thioformaldehyde. It consists of a six-membered ring with alternating methylene bridges and thioether groups. It is prepared by treatment of formaldehyde with hydrogen sulfide.

Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated; one (low-yielding) route is oxidation of a dithiolene complex. 3,4-Bis(trifluoromethyl)-1,2-dithiete is a particularly stable example.

Dithietanes are saturated heterocyclic compounds that contain two divalent sulfur atoms and two sp3-hybridized carbon centers. Two isomers are possible for this class of organosulfur compounds:

Sulfene is an extremely reactive chemical compound with the formula H2C=SO2. It is the simplest member of the sulfenes, the group of compounds which are S,S-dioxides of thioaldehydes and thioketones, and have the general formula R2C=SO2.
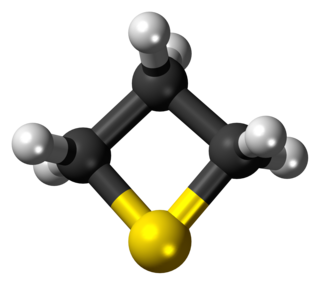
Thietane is a heterocyclic compound containing a saturated four-membered ring with three carbon atoms and one sulfur atom.
1,4,2-Dithiazole is a heterocyclic compound consisting of an unsaturated five-membered ring containing two carbon atoms, one nitrogen atom, and two sulfur atoms. 1,4,2-Dithiazole compounds may be formed by the reaction of nitrile sulfide with various reactive species; for instance thiocarbonyls via a 1,3-dipolar cycloaddition reaction. These compounds may be protonated by strong acids to give synthetically useful aromatic cations.
Thiirene is an organosulfur compound with the formula C2H2S. It can be viewed as a derivative of cyclopropene, but with the methylene group replaced by sulfur. It is antiaromatic and very labile.

1,2-Dithietane is a dithietane. It is a heterocyclic compound with a four-membered ring. Two sulfur atoms are adjacent, and the molecule is saturated. 1,2-Dithietane has not been produced as of 2000. The combination of ring strain, and lone pairs of electrons, which repel each other, on the sulfur atoms makes the sulfur-sulfur bond too weak to produce the molecule. However a few derivatives are known. 3,4-Diethyl-1,2-dithietane 1,1-dioxide has one sulfur fully oxidised. Dithiatopazine is a tricyclic compound with the -S-S- as a bridge. 1,2-Dithietan-3-one, the ketone of 1,2-dithietane, was produced in 2008 by reacting α-dithiolactone with ethoxycarbonylformonitrile oxide. 4,4-di-tert-butyl-1,2-dithietan-3-one and the spiro compound 5,5,9,9-tetramethyl-1,2-dithiaspiro[3.5]nonan-3-one have also been made.
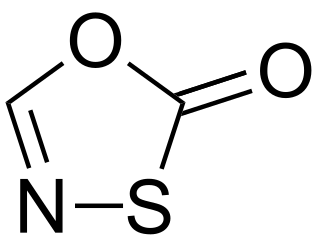
The oxathiazolones are a family of heterocyclic compounds in which the parent derivative has the molecular formula C2HNO2S and for which multiple isomers are known. The two known isomers with the highest profile in the literature are 1,3,4-oxathiazol-2-one and 1,4,2-oxathiazol-5-one.
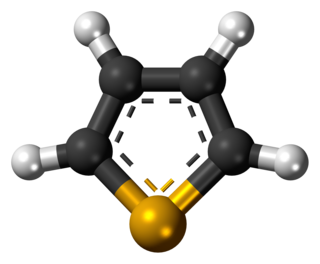
Selenophene is an organic compound with the chemical formula C4H4Se. It is an unsaturated compound containing a five-member ring with four carbon atoms and one selenium atom. It is a selenium analog of furan C4H4O and thiophene C4H4S. A colorless liquid, it is one of the more common selenium heterocycles.
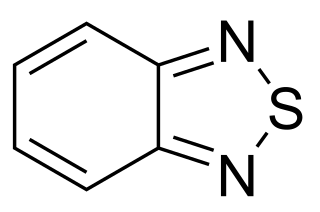
2,1,3-Benzothiadiazole is a bicyclic molecule composed of a benzene ring that is fused to a 1,2,5-thiadiazole.














