
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza; drugs which inhibit growth of viruses are termed antiviral drugs or antivirals rather than antibiotics. They are also not effective against fungi; drugs which inhibit growth of fungi are called antifungal drugs.

Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is, resistance has evolved. Antimicrobial resistance and antineoplastic resistance challenge clinical care and drive research. When an organism is resistant to more than one drug, it is said to be multidrug-resistant.

Linezolid is an antibiotic used for the treatment of infections caused by Gram-positive bacteria that are resistant to other antibiotics. Linezolid is active against most Gram-positive bacteria that cause disease, including streptococci, vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA). The main uses are infections of the skin and pneumonia although it may be used for a variety of other infections including drug-resistant tuberculosis. It is used either by injection into a vein or by mouth.

Sulfonamide is a functional group that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides.
Bone marrow suppression also known as myelotoxicity or myelosuppression, is the decrease in production of cells responsible for providing immunity (leukocytes), carrying oxygen (erythrocytes), and/or those responsible for normal blood clotting (thrombocytes). Bone marrow suppression is a serious side effect of chemotherapy and certain drugs affecting the immune system such as azathioprine. The risk is especially high in cytotoxic chemotherapy for leukemia. In the case of non-small-cell lung cancer, myelosuppression predisposition was shown to be modulated by enhancer mutations.

The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium.

Carbenicillin is a bactericidal antibiotic belonging to the carboxypenicillin subgroup of the penicillins. It was discovered by scientists at Beecham and marketed as Pyopen. It has Gram-negative coverage which includes Pseudomonas aeruginosa but limited Gram-positive coverage. The carboxypenicillins are susceptible to degradation by beta-lactamase enzymes, although they are more resistant than ampicillin to degradation. Carbenicillin is also more stable at lower pH than ampicillin.
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used in chemotherapy for cancer.

Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen Pseudomonas aeruginosa. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin".

Hetacillin is a beta-lactam antibiotic that is part of the aminopenicillin family. It is a prodrug and has no antibacterial activity itself, but quickly splits off acetone in the human body to form ampicillin, which is active against a variety of bacteria.

Imipenem is a synthetic β-lactam antibiotic belonging to the carbapenems chemical class. developed by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus playing a key role in the treatment of infections not readily treated with other antibiotics. It is usually administered through intravenous injection.

'Novobiocin, also known as albamycin or ', is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Temocillin is a β-lactamase-resistant penicillin introduced by Beecham, marketed by Eumedica Pharmaceuticals as Negaban. It is used primarily for the treatment of multiple drug-resistant, Gram-negative bacteria.
It is a 6-methoxy penicillin; it is also a carboxypenicillin.

Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin.

Nigericin is an antibiotic derived from Streptomyces hygroscopicus. Its isolation from soil from Nigeria was described in the 1950s, by R.L Harned, and in 1968 the structure could be elucidated by X-ray crystallography. The structure and properties of nigericin are similar to the antibiotic monensin. Commercially it is obtained as a byproduct, or contaminant, at the fermentation of geldanamycin. It is also called polyetherin A, azalomycin M, helixin C, antibiotic K178, and antibiotic X-464.

Sulfamethizole is a sulfonamide antibiotic.
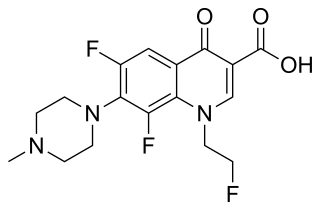
Fleroxacin is a quinolone antibiotic. It is sold under the brand names Quinodis and Megalocin.

Polypeptide antibiotics are a chemically diverse class of anti-infective and antitumor antibiotics containing non-protein polypeptide chains. Examples of this class include actinomycin, bacitracin, colistin, and polymyxin B. Actinomycin-D has found use in cancer chemotherapy. Most other polypeptide antibiotics are too toxic for systemic administration, but can safely be administered topically to the skin as an antiseptic for shallow cuts and abrasions.

Mecillinam (INN) or amdinocillin (USAN) is an extended-spectrum penicillin antibiotic of the amidinopenicillin class that binds specifically to penicillin binding protein 2 (PBP2), and is only considered to be active against Gram-negative bacteria. It is used primarily in the treatment of urinary tract infections, and has also been used to treat typhoid and paratyphoid fever. Because mecillinam has very low oral bioavailability, an orally active prodrug was developed: pivmecillinam.

Helicobacter cinaedi is a bacterium in the family Helicobacteraceae, Campylobacterales order, Helicobacteraceae family, Helicobacter genus. It was formerly known as Campylobacter cinaedi until molecular analysis published in 1991 led to a major revision of the genus Campylobacter. H. cinaedi is a curved, spiral, or fusiform rod with flagellum at both of its ends which it uses to dart around. The bacterium is a pathogen.


















