An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely-used stain in electron microscopy.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Transition metal dinitrogen complexes are coordination compounds that contain transition metals as ion centers the dinitrogen molecules (N2) as ligands.
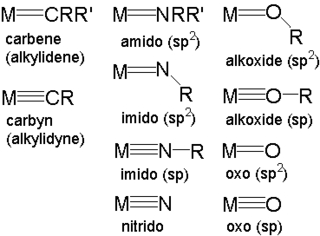
In organometallic chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. Transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds are molecular, i.e. discrete in contrast to the polymeric, dense nitride materials that are useful in materials science. The distinction between the molecular and solid-state polymers is not always very clear as illustrated by the materials Li6MoN4 and more condensed derivatives such as Na3MoN3. Transition metal nitrido complexes have attracted interest in part because it is assumed that nitrogen fixation proceeds via nitrido intermediates. Nitrido complexes have long been known, the first example being salts of [OsO3N]−, described in the 19th century.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.

Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amido complexes of the parent amido ligand NH2− are rare compared to complexes with diorganylamido ligand, such as dimethylamido. Amide ligands have two electron pairs available for bonding.
Phosphinoimidates, also known as phophinimides, are the anions derived from phosphine imides with the structure [R3P=N]− (R = alkyl or aryl). Phosphinimide ligands are used to for transition metal complexes that are highly active catalysts in some olefin polymerization reactions.
Diiminopyridines are a class of diimine ligands. They featuring a pyridine nucleus with imine sidearms appended to the 2,6–positions. The three nitrogen centres bind metals in a tridentate fashion, forming pincer complexes. Diiminopyridines are notable as non-innocent ligand that can assume more than one oxidation state. Complexes of DIPs participate in a range of chemical reactions, including ethylene polymerization, hydrosilylation, and hydrogenation.

trans-Bis(dinitrogen)bis[1,2-bis(diphenylphosphino)ethane]molybdenum(0) is a coordination complex with the formula Mo(N2)2(dppe)2. It is a relatively air stable yellow-orange solid. It is notable as being the first discovered dinitrogen containing complex of molybdenum.

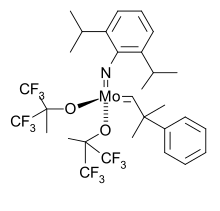
2,6-Diisopropylaniline is an organic compound with the formula H2NC6H3(CHMe2)2 (Me = CH3). It is a colorless liquid although, like many anilines, samples can appear yellow or brown. 2,6-Diisopropylaniline is a bulky aromatic amine that is often used to make ligands in coordination chemistry. The Schrock carbenes often are transition metal imido complexes derived from this aniline. Condensation with diacetylpyridine and acetylacetone gives, respectively, diiminopyridine and NacNac ligands.
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
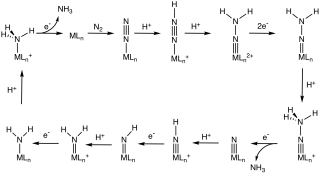
Abiological nitrogen fixation describes chemical processes that fix (react with) N2, usually with the goal of generating ammonia. The dominant technology for abiological nitrogen fixation is the Haber process, which uses an iron-based heterogeneous catalysts and H2 to convert N2 to NH3. This article focuses on homogeneous (soluble) catalysts for the same or similar conversions.

In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.