
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis."
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5O)3SiH). Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).
Reductions with hydrosilanes are methods used for hydrogenation and hydrogenolysis of organic compounds. The approach is a subset of ionic hydrogenation. In this particular method, the substrate is treated with a hydrosilane and auxiliary reagent, often a strong acid, resulting in formal transfer of hydride from silicon to carbon. This style of reduction with hydrosilanes enjoys diverse if specialized applications.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds has provided insights into the mechanisms of migratory insertion reactions.
The dehydrogenative coupling of silanes is a reaction type for the formation of Si-Si bonds. Although never commercialized, the reaction has been demonstrated for the synthesis of certain disilanes as well as polysilanes. These reactions generally require catalysts.

In chemistry, a sigma complex or σ-complex usually refers to a family of coordination complexes where one or more ligand interacts with the metal using the bonding electrons in a sigma bond. Dihydrogen complexes are examples. Transition metal silane complexes are often especially stable sigma complexes. A subset of sigma complexes are agostic complexes, where a C-H sigma bond functions as the donor ligand. In some cases, even C-C bonds function as sigma ligands. Sigma complexes are of great mechanistic significance, despite their frequent fragility. They represent an initial interaction between the metal center and saturated substrates. Sigma complexes are generally assumed to be intermediates prior to full oxidative addition.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
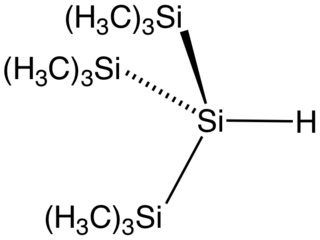
Tris(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)3SiH (where Me = CH3). It is a colorless liquid that is classified as a hydrosilane since it contains an Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in trimethylsilane is 94 kcal/mol. With such a weak bond, the compound is used as a reagent to deliver hydrogen atoms. The compound has been described as an environmentally benign analogue of tributyltin hydride.

Transition metal complexes of aldehydes and ketones describes coordination complexes with aldehyde (RCHO) and ketone (R2CO) ligands. Because aldehydes and ketones are common, the area is of fundamental interest. Some reactions that are useful in organic chemistry involve such complexes.

Transition metal acyl complexes describes organometallic complexes containing one or more acyl (RCO) ligands. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.

A silanide is a chemical compound containing an anionic silicon(IV) centre, the parent ion being SiH−3. The hydrogen atoms can also be substituted to produce more complex derivative anions such as tris(trimethylsilyl)silanide (hypersilyl), tris(tert-butyl)silanide, tris(pentafluoroethyl)silanide, or triphenylsilanide. The simple silanide ion can also be called trihydridosilanide or silyl hydride.
Phosphanides are chemicals containing the [PH2]− anion. This is also known as the phosphino anion or phosphido ligand. The IUPAC name can also be dihydridophosphate(1−).
The stabilization of bismuth's +3 oxidation state due to the inert pair effect yields a plethora of organometallic bismuth-transition metal compounds and clusters with interesting electronics and 3D structures.
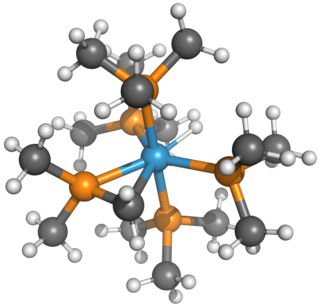
Pentakis(trimethylphosphine)tungsten (W(PMe3)5, Me=CH3) and its physically relevant "tucked-in" isomer, [(dimethylphosphino-κP)methyl-κC]hydrotetrakis(trimethylphosphine)tungsten (W(PMe3)4(η2-CH2PMe2)H), are organotungsten complexes. Formally, the former is a tungsten(0) complex, whereas the latter is a tungsten(II) complex. W(PMe3)4(η2-CH2PMe2)H's tungsten center is electron-rich and, thus, prone to oxidation. W(PMe3)4(η2-CH2PMe2)H has been used as a starting retron for some challenging chemistry, such as C-C bond activation, tungsten-chalcogenide multiple bonding, tungsten-tetrel multiple bonding, and desulfurization.












