Bioleaching is the extraction or liberation of metals from their ores through the use of living organisms. Bioleaching is one of several applications within biohydrometallurgy and several methods are used to treat ores or concentrates containing copper, zinc, lead, arsenic, antimony, nickel, molybdenum, gold, silver, and cobalt.

In chemistry, cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.

A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Silver is a chemical element; it has symbol Ag ) and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form, as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining.

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".

Thiocyanates are salts containing the thiocyanate anion [SCN]−. [SCN]− is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.

Silver chloride is an inorganic chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite.
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms Ps–Ps or Ps–X, such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide.
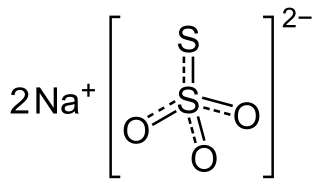
Sodium thiosulfate is an inorganic compound with the formula Na2S2O3·(H2O)(x). Typically it is available as the white or colorless pentahydrate. It is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications.
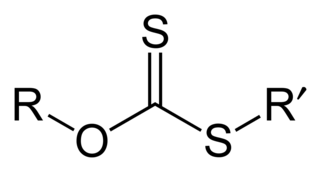
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Thiosulfate is an oxyanion of sulfur with the chemical formula S2O2−3. Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, such as sodium thiosulfate Na2S2O3 and ammonium thiosulfate (NH4)2S2O3. Thiosulfate salts occur naturally. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances.

A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions.

Silver is a relatively unreactive metal, although it can form several compounds. The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass. Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.

Sodium aurothiosulfate, or sanocrysin, is the inorganic compound with the formula Na3[Au(S2O3)2]·2H2O. It is the trisodium salt of the coordination complex of gold(I), [Au(S2O3)2]3−. The dihydrate, which is colorless, crystallizes with two waters of crystallization. The compound has some medicinal properties as well as potential for hydrometallurgy.

Thiosulfate dehydrogenase is an enzyme that catalyzes the chemical reaction:
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands. Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. The resulting cyanometallate anions are often used as building blocks for more complex structures called coordination polymers, the best known example of which is Prussian blue, a common dyestuff.
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand, but of course many complexes are known to consist of a mix of aquo and other ligands.

Ammonium thiosulfate is an inorganic compound with the formula [NH4]2S2O3. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether.
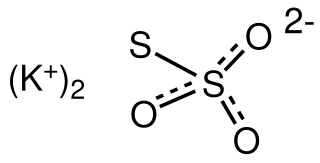
Potassium thiosulfate, commonly abbreviated KTS, is an inorganic compound with the formula K2S2O3. This salt can form multiple hydrates, such as the monohydrate, dihydrate, and the pentahydrate, all of which are white or colorless solids. It is used as a fertilizer.
















