Structure and bonding
Hard metal cations, as classified by HSAB theory, tend to form N-bonded complexes (isothiocyanates), whereas class B or soft metal cations tend to form S-bonded thiocyanate complexes. For the isothiocyanates, the M-N-C angle is usually close to 180°. For the thiocyanates, the M-S-C angle is usually close to 100°.
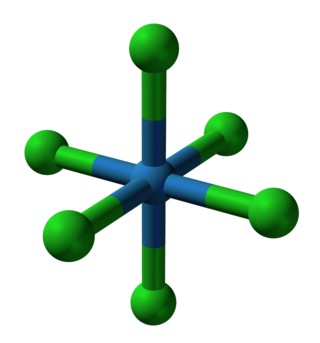
- Crystal structure of [NiII(NCS)6]4-, a homoleptic complex of six isothiocyanate ligands. Color code: blue = N, yellow = S.
- Structure of Pd(Me2N(CH2)3PPh2)(SCN)(NCS) illustrating linkage isomerism of the SCN- ligand. [2]
- Crystal structure of [ReIV(NCS)5(SCN)]2-. [3] Color code: blue = N, yellow = S.
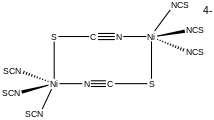
- Structure of the dinuclear complex [NiII2(SCN)8]4- with a bridging SCN- ligand.
Homoleptic complexes
Most homoleptic complexes of NCS- feature isothiocyanate ligands (N-bonded). All first-row metals bind thiocyanate in this way. [4] Octahedral complexes [M(NCS)6]z- include M = Ti(III), Cr(III), Mn(II), Fe(III), Ni(II), Mo(III), Tc(IV), and Ru(III). [5] Four-coordinated tetrakis(isothiocyanate) complexes would be tetrahedral since isothiocyanate is a weak-field ligand. Two examples are the deep blue [Co(NCS)4]2- and the green [Ni(NCS)4]2-. [6]
Few homoleptic complexes of NCS- feature thiocyanate ligands (S-bonded). Octahedral complexes include [M(SCN)6]3- (M = Rh [7] and Ir [8] ) and [Pt(SCN)6]2-. Square planar complexes include [M(SCN)4]z- (M = Pd(II), Pt(II), [9] and Au(III)). Colorless [Hg(SCN)4]2- is tetrahedral.
Some octahedral isothiocyanate complexes undergo redox reactions reversibly. Orange [Os(NCS)6]3- can be oxidized to violet [Os(NCS)6]2-. The Os-N distances in both derivatives are almost identical at 200 picometers. [10]
Linkage isomerism
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. [11] Thiocyanate can bind metals at either sulfur or nitrogen — it is an ambidentate ligand. Other factors, e.g. kinetics and solubility, sometimes influence the observed isomer. For example, [Co(NH3)5(NCS)]2+ is the thermodynamic isomer, but [Co(NH3)5(SCN)]2+ forms as the kinetic product of the reaction of thiocyanate salts with [Co(NH3)5(H2O)]3+. [12]
- [Co(NH3)5(H2O)]3+ + SCN− → [Co(NH3)5(SCN)]2+ + H2O
- [Co(NH3)5(SCN)]2+ → [Co(NH3)5(NCS)]2+
Some complexes of SCN- feature both but only thiocyanate and isothiocyanate ligands. Examples are found for heavy metals in the middle of the d-period: Ir(III), [13] and Re(IV). [3]
SCN-bridged complexes
As a ligand, [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). One example of an SCN-bridged complex is [Ni2(SCN)8]4-. [6]
Mixed ligand complexes
This article focuses on homoleptic complexes, which are simpler to describe and analyze. Most complexes of SCN-, however are mixed ligand species. Mentioned above is one example, [Co(NH3)5(NCS)]2+. Another example is [OsCl2(SCN)2(NCS)2]2-. [14] Reinecke's salt, a precipitating agent, is a derivative of [Cr(NCS)4(NH3)2]-.