
Paint is a liquid pigment that, after applied to a solid material and allowed to dry, adds a film-like layer, in most cases to create an image, known as a painting. Paint can be made in many colors and types. Most paints are either oil-based or water-based, and each has distinct characteristics.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.
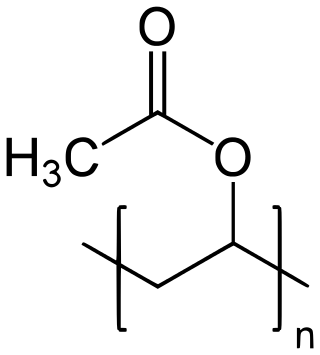
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue, PVA glue, white glue, carpenter's glue, school glue, or Elmer's Glue in the US, is a widely available adhesive used for porous materials like wood, paper, and cloth. An aliphatic rubbery synthetic polymer with the formula (C4H6O2)n, it belongs to the polyvinyl ester family, with the general formula −[RCOOCHCH2]−. It is a type of thermoplastic.
In polymer chemistry, emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomers, and surfactants. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Polyvinyl fluoride (PVF) or –(CH2CHF)n– is a polymer material mainly used in the flammability-lowering coatings of airplane interiors and photovoltaic module backsheets. It is also used in raincoats and metal sheeting. Polyvinyl fluoride is a thermoplastic fluoropolymer with a repeating vinyl fluoride unit, and it is structurally very similar to polyvinyl chloride.

Poly(vinyl alcohol) (PVOH, PVA, or PVAl) is a water-soluble synthetic polymer. It has the idealized formula [CH2CH(OH)]n. It is used in papermaking, textile warp sizing, as a thickener and emulsion stabilizer in polyvinyl acetate (PVAc) adhesive formulations, in a variety of coatings, and 3D printing. It is colourless (white) and odorless. It is commonly supplied as beads or as solutions in water. Without an externally added crosslinking agent, PVA solution can be gelled through repeated freezing-thawing, yielding highly strong, ultrapure, biocompatible hydrogels which have been used for a variety of applications such as vascular stents, cartilages, contact lenses, etc.

Ethylene-vinyl acetate (EVA), also known as poly(ethylene-vinyl acetate) (PEVA), is a copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 50%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used.

Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
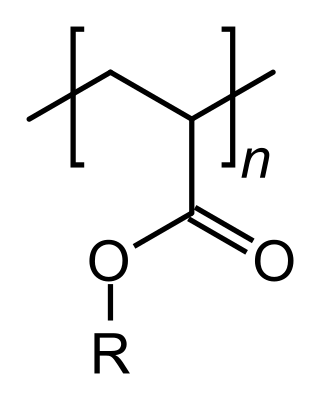
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.

An acrylic resin is a thermoplastic or thermosetting plastic substance typically derived from acrylic acid, methacrylic acid and acrylate monomers such as butyl acrylate and methacrylate monomers such as methyl methacrylate. Thermoplastic acrylics designate a group of acrylic resins typically containing both a high molecular weight and a high glass transition temperature which exhibit lacquer dry capability. Acrylic resins designed for use in two component systems for crosslinking with isocyanate are referred to as polyols and are made with the monomers previously mentioned as well as hydroxy monomers such as hydroxy ethyl methacrylate. Acrylic resins are produced in different liquid carriers such as a hydrocarbon solvent or water in which case they are referred to as emulsions or dispersions and they are also provided in 100% solids bead form.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.
Dimethylaminoethyl acrylate or DMAEA is an unsaturated carboxylic acid ester having a tertiary amino group. It is a colorless to yellowish, water-miscible liquid with a pungent, amine-like odor. DMAEA is an important acrylic monomer that gives basic properties to copolymers.
Waterborne resins are sometimes called water-based resins. They are resins or polymeric resins that use water as the carrying medium as opposed to solvent or solvent-less. Resins are used in the production of coatings, adhesives, sealants, elastomers and composite materials. When the phrase waterborne resin is used, it usually describes all resins which have water as the main carrying solvent. The resin could be water-soluble, water reducible or water dispersed.















