Related Research Articles

In all bilaterian animals, the mesoderm is one of the three primary germ layers in the very early embryo. The other two layers are the ectoderm and endoderm, with the mesoderm as the middle layer between them.
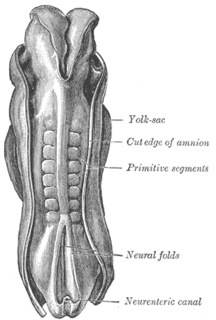
The somites are a set of bilaterally paired blocks of paraxial mesoderm that form in the embryonic stage of somitogenesis, along the head-to-tail axis in segmented animals. In vertebrates, somites subdivide into the sclerotomes, myotomes, syndetomes and dermatomes that give rise to the vertebrae of the vertebral column, rib cage and part of the occipital bone; skeletal muscle, cartilage, tendons, and skin.

Somitogenesis is the process by which somites form. Somites are bilaterally paired blocks of paraxial mesoderm that form along the anterior-posterior axis of the developing embryo in segmented animals. In vertebrates, somites give rise to skeletal muscle, cartilage, tendons, endothelium, and dermis.
The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.

Neural crest cells are a temporary group of cells unique to vertebrates that arise from the embryonic ectoderm germ layer, and in turn give rise to a diverse cell lineage—including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia.

Intermediate mesoderm or intermediate mesenchyme is a narrow section of the mesoderm located between the paraxial mesoderm and the lateral plate of the developing embryo. The intermediate mesoderm develops into vital parts of the urogenital system, as well as the reproductive system.

The apical ectodermal ridge (AER) is a structure that forms from the ectodermal cells at the distal end of each limb bud and acts as a major signaling center to ensure proper development of a limb. After the limb bud induces AER formation, the AER and limb mesenchyme—including the zone of polarizing activity (ZPA)—continue to communicate with each other to direct further limb development.
Mesenchyme is a type of connective tissue found mostly during the embryonic development of bilateral triploblast animals.
The limb bud is a structure formed early in vertebrate limb development. As a result of interactions between the ectoderm and underlying mesoderm, formation occurs roughly around the fourth week of development. In the development of the human embryo the upper limb bud appears in the third week and the lower limb bud appears four days later.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

WNT4 is a secreted protein that in humans is encoded by the Wnt4 gene, found on chromosome 1. It promotes female sex development and represses male sex development. Loss of function can have serious consequences, such as female to male sex reversal.

Proto-oncogene protein Wnt-1 is a protein that in humans is encoded by the WNT1 gene.

Protein odd-skipped-related 1 is a transcription factor that in humans is encoded by the OSR1 gene. The OSR1 and OSR2 transcription factors participate in the normal development of body parts such as the kidney.

Protein Wnt-10b is a protein that in humans is encoded by the WNT10B gene.

Protein Wnt-9a is a protein that in humans is encoded by the WNT9A gene.

Wnt7b is a signaling protein that plays a crucial role for many developmental processes including placental, lung, eye, dendrite, and bone formation along with kidney development. The primary role of Wnt7b is to establish the cortico-medullary axis of epithelial organization.

Wnt-10a is a protein that in humans is encoded by the WNT10A gene.
Neural crest cells are multipotent cells required for the development of cells, tissues and organ systems. A subpopulation of neural crest cells are the cardiac neural crest complex. This complex refers to the cells found amongst the midotic placode and somite 3 destined to undergo epithelial-mesenchymal transformation and migration to the heart via pharyngeal arches 3, 4 and 6.
The clock and wavefront model is a model used to describe the process of somitogenesis in vertebrates. Somitogenesis is the process by which somites, blocks of mesoderm that give rise to a variety of connective tissues, are formed.

Myogenic factor 5 is a protein that in humans is encoded by the MYF5 gene. It is a protein with a key role in regulating muscle differentiation or myogenesis, specifically the development of skeletal muscle. Myf5 belongs to a family of proteins known as myogenic regulatory factors (MRFs). These basic helix loop helix transcription factors act sequentially in myogenic differentiation. MRF family members include Myf5, MyoD (Myf3), myogenin, and MRF4 (Myf6). This transcription factor is the earliest of all MRFs to be expressed in the embryo, where it is only markedly expressed for a few days. It functions during that time to commit myogenic precursor cells to become skeletal muscle. In fact, its expression in proliferating myoblasts has led to its classification as a determination factor. Furthermore, Myf5 is a master regulator of muscle development, possessing the ability to induce a muscle phenotype upon its forced expression in fibroblastic cells.
References
- 1 2 "Entrez Gene: WNT2 wingless-type MMTV integration site family, member 2".
- ↑ Rankin J, Strachan T, Lako M, Lindsay S (1999). "Partial cloning and assignment of WNT6 to human chromosome band 2q35 by in situ hybridization". Cytogenet. Cell Genet. 84 (1–2): 50–2. doi:10.1159/000015212. PMID 10343101. S2CID 19452847.
- ↑ Kirikoshi H, Sekihara H, Katoh M (May 2001). "WNT10A and WNT6, clustered in human chromosome 2q35 region with head-to-tail manner, are strongly coexpressed in SW480 cells". Biochem. Biophys. Res. Commun. 283 (4): 798–805. doi:10.1006/bbrc.2001.4855. PMID 11350055.
- 1 2 3 Lavery, Danielle L.; Martin, Jennifer; Turnbull, Yvonne D.; Hoppler, Stefan (November 2008). "Wnt6 signaling regulates heart muscle development during organogenesis". Developmental Biology. 323 (2): 177–188. doi:10.1016/j.ydbio.2008.08.032. PMC 2593796 . PMID 18804460.
- ↑ Zhang, Lingling; Li, Hanjun; Yu, Jian; Cao, Jingjing; Chen, Huihui; Zhao, Haixia (March 2014). "Ectodermal Wnt signaling regulates abdominal myogenesis during ventral body wall development". Developmental Biology. 387 (1): 64–72. doi: 10.1016/j.ydbio.2013.12.027 . PMID 24394376.
- 1 2 3 4 Schmidt, Corina; Stoeckelhuber, Mechthild; McKinnell, Iain; Putz, Reinhard; Christ, Bodo; Patel, Ketan (July 2004). "Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation". Developmental Biology. 271 (1): 198–209. doi:10.1016/j.ydbio.2004.03.016. PMID 15196961.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
| This article on a gene on human chromosome 2 is a stub. You can help Wikipedia by expanding it. |