
Zylofuramine is a stimulant drug. It was developed in 1961, and was intended for use as an appetite suppressant and for the treatment of senile dementia in the elderly, but there is little information about it and it does not appear to have ever been marketed.

4'-Methyl-α-pyrrolidinohexiophenone (MPHP) is a stimulant compound which has been reported as a novel designer drug. It is closely related to pyrovalerone, being simply its chain-lengthened homologue. In the pyrrolidinophenone series, stimulant activity is maintained so long as the positions of the aryl, ketone and pyrrolidinyl groups are held constant, while the alkyl backbone can be varied anywhere between three and as many as seven carbons, with highest potency usually seen with the pentyl or isohexyl backbone, and a variety of substituents are tolerated on the aromatic ring.

Eutylone is a stimulant and empathogenic compound developed in the 1960s, which is classified as a designer drug. It was first reported to the EMCDDA in 2014 and became widespread internationally in 2019-2020 following bans on the related compound ephylone. It is a synthetic cathinone. In 2021, eutylone was the most common cathinone identified by the Drug Enforcement Administration in the United States.
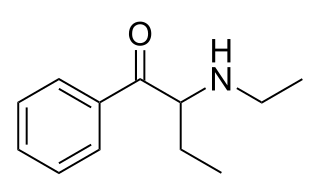
N-Ethylbuphedrone is a stimulant of the cathinone class that has been sold as a designer drug. It is the β-ketone analogue of N,alpha-diethylphenylethylamine.

3-Fluorophenmetrazine is a phenylmorpholine-based stimulant and fluorinated analogue of phenmetrazine that has been sold online as a designer drug.

α-Pyrrolidinohexiophenone is a synthetic stimulant drug of the cathinone class developed in the 1960s which has been reported as a novel designer drug.

4-Chloro-alpha-pyrrolidinovalerophenone is an emerging recreational designer drug of the pyrrolidinophenone class, similar in structure to alpha-pyrrolidinopentiophenone (α-PVP). The pharmacology and toxicity of this compound is unknown, though it is presumed to be a stimulant drug.
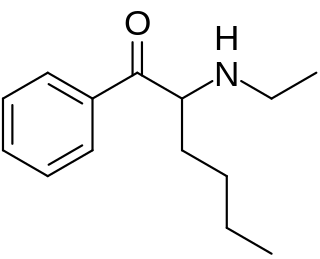
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.

MDPHP (3',4'-Methylenedioxy-α-pyrrolidinohexiophenone) is a stimulant of the cathinone class originally developed in the 1960s, which has been reported as a novel designer drug. In the UK its slang name is monkey dust. It is closely related to the potent stimulant MDPV though with slightly milder effects, and has been used as an alternative in some countries following the banning of MDPV.

α-Pyrrolidinoheptaphenone is a designer drug of the pyrrolidinophenone class of cathinones. It is the higher homolog of α-pyrrolidinohexiophenone (α-PHP).

4-Methyl-α-ethylaminopentiophenone (4-MEAP) is a designer drug of the cathinone class. It is a higher homolog of 4-methylpentedrone (4-MPD) with an ethyl group in place of the methyl group. 4-MEAP has been found in samples of drugs sold as 4-MPD.

α-Pyrrolidinoisohexanophenone is a stimulant drug of the cathinone class that has been sold online as a designer drug. It is a positional isomer of pyrovalerone, with the methyl group shifted from the 4-position of the aromatic ring to the 4-position of the acyl chain. In a classic 2006 study of pyrrolidinyl cathinone derivatives by Meltzer et al. at Organix, the alpha-isobutyl derivative of pyrovalerone, O-2494, was found to have the highest potency in vitro as an inhibitor of the dopamine transporter of the alpha substituted derivatives tested. α-PiHP acts as a norepinephrine–dopamine reuptake inhibitor. Compared to α-PVP and α-PHP, α-PiHP displays a higher selectivity for the dopamine transporter. Similar to other cathinones, use of α-PiHP can result in compulsive redosing, addiction, anxiety, paranoia, and psychosis. In July 2016 α-PHiP was first identified as a designer drug when it was reported to the EMCDDA by a forensic laboratory in Slovenia.

3F-PiHP (3F-α-PiHP) is a recreational designer drug from the substituted cathinone family, with stimulant effects. It was first identified in both Sweden and Finland in mid-2019, and was made illegal in Finland in August 2019.
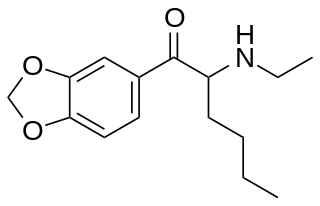
N-Ethylhexylone is a recreational designer drug from the substituted cathinone family, with stimulant effects. It was first identified in Poland in August 2019. It is illegal in Taiwan since July 2020, where it had been sold mixed with plant material under the name 彩虹菸.
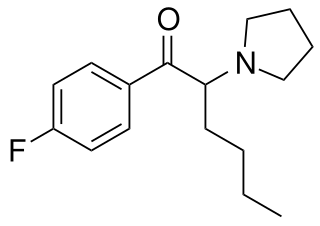
4-Fluoro-alpha-PHP (4F-PHP) is a recreational designer drug from the substituted cathinone family with stimulant effects, which first appeared on the illicit market in around 2017.
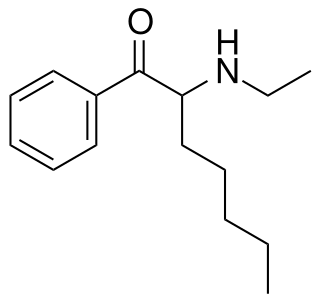
N-Ethylheptedrone is a recreational designer drug from the substituted cathinone family, with stimulant effects. It is a homologue of related drugs such as ethcathinone, N-ethylbuphedrone and N-ethylhexedrone but with a longer pentyl side chain. It was first identified in Hungary in 2019, and has since been reported in New Zealand.
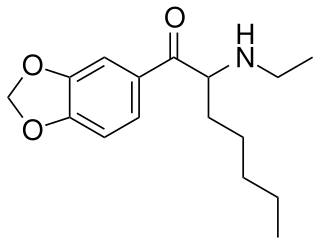
N-Ethylheptylone (HEP) is a recreational designer drug from the substituted cathinone family, with stimulant effects. It is a homologue of related drugs such as ethylone, eutylone, ephylone and N-ethylhexylone but with a longer heptyl side chain. It was first reported in Sweden in 2019.

3-Fluoro-N-ethylbuphedrone (3F-NEB) is a substituted cathinone derivative with stimulant effects which has been sold as a designer drug. It was first identified in Sweden in 2021.
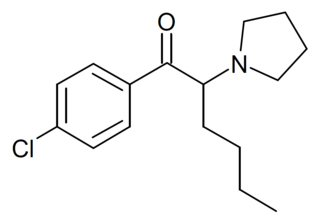
4-Chloro-alpha-Pyrrolidinohexiophenone (4-Cl-PHP) is a substituted cathinone derivative with stimulant effects, which has been sold as a designer drug. It was first officially identified by forensic laboratories in 2016, though anecdotal reports suggest it may have been available several years prior to this.



















