Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin clathrum meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection.

SNARE proteins — "SNAPREceptor" — are a large protein family consisting of at least 24 members in yeasts and more than 60 members in mammalian cells. The primary role of SNARE proteins is to mediate vesicle fusion – the fusion of vesicles with the target membrane; this notably mediates exocytosis, but can also mediate the fusion of vesicles with membrane-bound compartments. The best studied SNAREs are those that mediate the neurotransmitter release of synaptic vesicles in neurons. These neuronal SNAREs are the targets of the neurotoxins responsible for botulism and tetanus produced by certain bacteria.
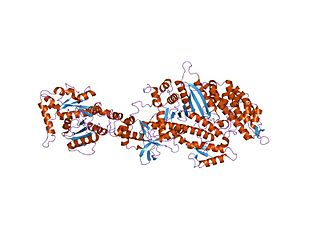
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamin is part of the "dynamin superfamily", which includes classical dynamins, dynamin-like proteins, Mx proteins, OPA, mitofusins, and GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface as well as at the Golgi apparatus. Dynamin family members also play a role in many processes including division of organelles, cytokinesis and microbial pathogen resistance.
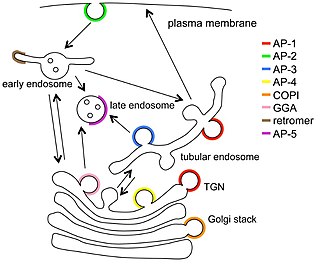
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

An alpha solenoid is a protein fold composed of repeating alpha helix subunits, commonly helix-turn-helix motifs, arranged in antiparallel fashion to form a superhelix. Alpha solenoids are known for their flexibility and plasticity. Like beta propellers, alpha solenoids are a form of solenoid protein domain commonly found in the proteins comprising the nuclear pore complex. They are also common in membrane coat proteins known as coatomers, such as clathrin, and in regulatory proteins that form extensive protein-protein interactions with their binding partners. Examples of alpha solenoid structures binding RNA and lipids have also been described.
AP180 is a protein that plays an important role in clathrin-mediated endocytosis of synaptic vesicles. It is capable of simultaneously binding both membrane lipids and clathrin and is therefore thought to recruit clathrin to the membrane of newly invaginating vesicles. In Drosophila melanogaster, deletion of the AP180 homologue, leads to enlarged but much fewer vesicles and an overall decrease in transmitter release. In D. melanogaster it was also shown that AP180 is also required for either recycling vesicle proteins and/or maintaining the distribution of both vesicle and synaptic proteins in the nerve terminal. A ubiquitous form of the protein in mammals, CALM, is named after its association with myeloid and lymphoid leukemias where some translocations map to this gene. The C-terminus of AP180 is a powerful and specific inhibitor of clathrin-mediated endocytosis.
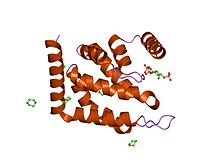
In molecular biology, BAR domains are highly conserved protein dimerisation domains that occur in many proteins involved in membrane dynamics in a cell. The BAR domain is banana-shaped and binds to membrane via its concave face. It is capable of sensing membrane curvature by binding preferentially to curved membranes. BAR domains are named after three proteins that they are found in: Bin, Amphiphysin and Rvs.

The ANTH domain is a membrane binding domain that shows weak specificity for PtdIns(4,5)P2. It was found in AP180 endocytotic accessory protein that has been implicated in the formation of clathrin-coated pits. The domain is involved in phosphatidylinositol 4,5-bisphosphate binding and is a universal adaptor for nucleation of clathrin coats.

Epsins are a family of highly conserved membrane proteins that are important in creating membrane curvature. Epsins contribute to membrane deformations like endocytosis, and block vesicle formation during mitosis.

Amphiphysin is a protein that in humans is encoded by the AMPH gene.

The AP2 adaptor complex is a multimeric protein that works on the cell membrane to internalize cargo in clathrin-mediated endocytosis. It is a stable complex of four adaptins which give rise to a structure that has a core domain and two appendage domains attached to the core domain by polypeptide linkers. These appendage domains are sometimes called 'ears'. The core domain binds to the membrane and to cargo destined for internalisation. The alpha and beta appendage domains bind to accessory proteins and to clathrin. Their interactions allow the temporal and spatial regulation of the assembly of clathrin-coated vesicles and their endocytosis.

AP-2 complex subunit alpha-2 is a protein that in humans is encoded by the AP2A2 gene.

Clathrin interactor 1 (CLINT1), also known as EPSIN4, is a protein which in humans is encoded by the CLINT1 gene.

Epsin-1 is a protein that in humans is encoded by the EPN1 gene.

Epsin-2 is a protein that in humans is encoded by the EPN2 gene.
Membrane curvature is the geometrical measure or characterization of the curvature of membranes. The membranes can be naturally occurring or man-made (synthetic). An example of naturally occurring membrane is the lipid bilayer of cells, also known as cellular membranes. Synthetic membranes can be obtained by preparing aqueous solutions of certain lipids. The lipids will then "aggregate" and form various phases and structures. According to the conditions and the chemical structures of the lipid, different phases will be observed. For instance, the lipid POPC tends to form lamellar vesicles in solution, whereas smaller lipids, such as detergents, will form micelles if the CMC is reached.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. These proteins are synthesized in the ribosomes, processed in the endoplasmic reticulum and transported from the Golgi apparatus to the trans-Golgi network, and from there via small carrier vesicles to their final destination compartment. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

The C-terminal domain ofBeta2-adaptin is a protein domain is involved in cell trafficking by aiding import and export of substances in and out of the cell.