Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested materials. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

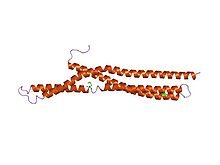
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle. The protein's name refers to this lattice structure, deriving from Latin clathri meaning lattice. Barbara Pearse named the protein clathrin at the suggestion of Graeme Mitchison, selecting it from three possible options. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection.
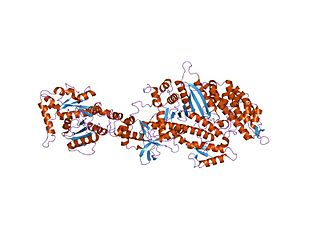
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamin is part of the "dynamin superfamily", which includes classical dynamins, dynamin-like proteins, Mx proteins, OPA1, mitofusins, and GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface as well as at the Golgi apparatus. Dynamin family members also play a role in many processes including division of organelles, cytokinesis and microbial pathogen resistance.
AP180 is a protein that plays an important role in clathrin-mediated endocytosis of synaptic vesicles. It is capable of simultaneously binding both membrane lipids and clathrin and is therefore thought to recruit clathrin to the membrane of newly invaginating vesicles. In Drosophila melanogaster, deletion of the AP180 homologue, leads to enlarged but much fewer vesicles and an overall decrease in transmitter release. In D. melanogaster it was also shown that AP180 is also required for either recycling vesicle proteins and/or maintaining the distribution of both vesicle and synaptic proteins in the nerve terminal. A ubiquitous form of the protein in mammals, CALM, is named after its association with myeloid and lymphoid leukemias where some translocations map to this gene. The C-terminus of AP180 is a powerful and specific inhibitor of clathrin-mediated endocytosis.
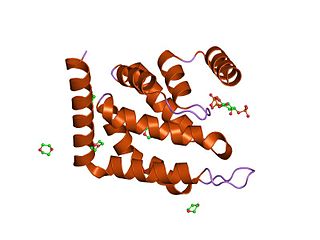
The epsin N-terminal homology (ENTH) domain is a structural domain that is found in proteins involved in endocytosis and cytoskeletal machinery.

Epsins are a family of highly conserved membrane proteins that are important in creating membrane curvature. Epsins contribute to membrane deformations like endocytosis, and block vesicle formation during mitosis.

Amphiphysin is a protein that in humans is encoded by the AMPH gene.
Synaptojanin is a protein involved in vesicle uncoating in neurons. This is an important regulatory lipid phosphatase. It dephosphorylates the D-5 position phosphate from phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and Phosphatidylinositol (4,5)-bisphosphate(PIP2). It belongs to family of 5-phosphatases, which are structurally unrelated to D-3 inositol phosphatases like PTEN. Other members of the family of 5'phosphoinositide phosphatases include OCRL, SHIP1, SHIP2, INPP5J, INPP5E, INPP5B, INPP5A and SKIP.

Myc box-dependent-interacting protein 1, also known as Bridging Integrator-1 and Amphiphysin-2 is a protein that in humans is encoded by the BIN1 gene.
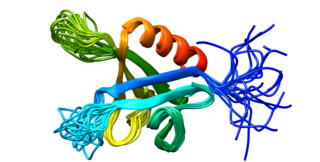
Dynamin-2 is a protein that in humans is encoded by the DNM2 gene.

Dynamin-1 is a protein that in humans is encoded by the DNM1 gene.

AP-2 complex subunit alpha-2 is a protein that in humans is encoded by the AP2A2 gene.

Endophilin-B1 is a protein that in humans is encoded by the SH3GLB1 gene. Endophilin-B1 belongs to the Bin/Amphiphysin/Rvs167 (BAR) family of proteins and plays a critical role in mitochondrial fission and fusion, as well as in autophagy and apoptosis. Loss of functional endophilin-B1 is seen in many different forms of cancer. The link between carcinogenesis and dysregulation of cell death pathways suggests that endophilin-B1 serves a critical tumor suppressor role in the cell, although the underlying mechanisms are not known.
Membrane curvature is the geometrical measure or characterization of the curvature of membranes. The membranes can be naturally occurring or man-made (synthetic). An example of naturally occurring membrane is the lipid bilayer of cells, also known as cellular membranes. Synthetic membranes can be obtained by preparing aqueous solutions of certain lipids. The lipids will then "aggregate" and form various phases and structures. According to the conditions and the chemical structures of the lipid, different phases will be observed. For instance, the lipid POPC tends to form lamellar vesicles in solution, whereas smaller lipids, such as detergents, will form micelles if the CMC was reached. There are five commonly proposed mechanisms by which membrane curvature is created, maintained, or controlled: lipid composition, shaped transmembrane proteins, protein motif insertion/BAR domains, protein scaffolding, and cytoskeleton scaffolding.
Bulk endocytosis refers to a form of endocytosis of synaptic vesicles at nerve terminals. In bulk endocytosis, compared to clathrin-mediated endocytosis, a larger area of presynaptic plasma membrane is internalised as cisternae or endosomes from which multiple synaptic vesicles can subsequently bud off. Bulk endocytosis is activated specifically during intense stimulation, such as during high-frequency trains of action potentials or in response to membrane depolarization by high extracellular concentrations of potassium.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.
SacI homology domain is most notably found at the amino terminal of the inositol 5'-phosphatase synaptojanin. Synaptic vesicles are recycled with remarkable speed and precision in nerve terminals. A major recycling pathway involves clathrin-mediated endocytosis at endocytic zones located around sites of release. Different 'accessory' proteins linked to this pathway have been shown to alter the shape and composition of lipid membranes, to modify membrane-coat protein interactions, and to influence actin polymerization. These include the GTPase dynamin, the lysophosphatidic acid acyl transferase endophilin, and the phosphoinositide phosphatase synaptojanin.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. These proteins are synthesized in the ribosomes, processed in the endoplasmic reticulum and transported from the Golgi apparatus to the trans-Golgi network, and from there via small carrier vesicles to their final destination compartment. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

The C-terminal domain ofBeta2-adaptin is a protein domain is involved in cell trafficking by aiding import and export of substances in and out of the cell.
Clathrin-independent endocytosis refers to the cellular process by which cells internalize extracellular molecules and particles through mechanisms that do not rely on the protein clathrin, playing a crucial role in diverse physiological processes such as nutrient uptake, membrane turnover, and cellular signaling.