
Bromocriptine, originally marketed as Parlodel and subsequently under many brand names, is an ergoline derivative and dopamine agonist that is used in the treatment of pituitary tumors, Parkinson's disease, hyperprolactinaemia, neuroleptic malignant syndrome, and, as an adjunct, type 2 diabetes.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
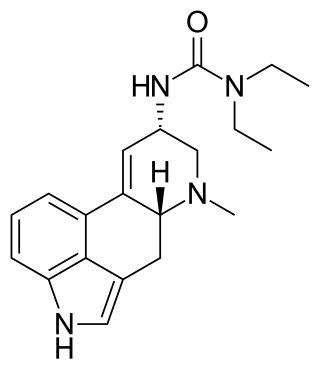
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.
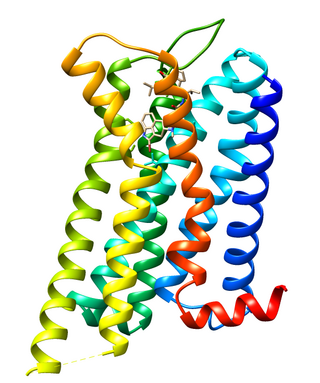
Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the DRD2 gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined.

The adenosine A2A receptor, also known as ADORA2A, is an adenosine receptor, and also denotes the human gene encoding it.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.
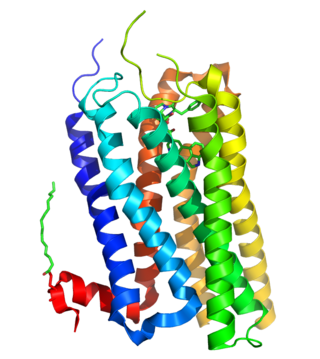
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

5'-Guanidinonaltrindole (5'-GNTI) is an opioid antagonist used in scientific research which is highly selective for the κ opioid receptor. It is 5x more potent and 500 times more selective than the commonly used κ-opioid antagonist norbinaltorphimine. It has a slow onset and long duration of action, and produces antidepressant effects in animal studies. It also increases allodynia by interfering with the action of the κ-opioid peptide dynorphin.
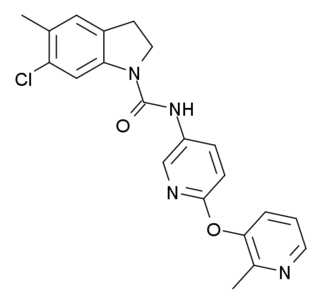
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

RS-102221 is a drug developed by Hoffmann–La Roche, which was one of the first compounds discovered that acts as a potent and selective antagonist at the serotonin 5-HT2C receptor, with around 100× selectivity over the closely related 5-HT2A and 5-HT2B receptors. It has anxiolytic effects in animal studies, increases the effectiveness of SSRI antidepressants, and shows a complex interaction with cocaine, increasing some effects but decreasing others, reflecting a role for the 5-HT2C receptor in regulation of the dopamine signalling system in the brain.
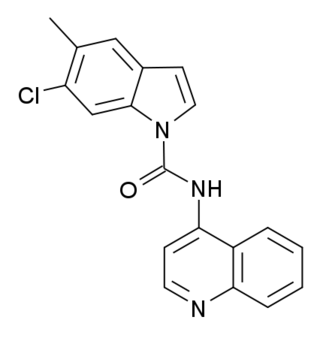
SB-215505 is a drug which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with good selectivity over the related 5-HT2A and 5-HT2C receptors. It is used in scientific research into the function of the 5-HT2 family of receptors, especially to study the role of 5-HT2B receptors in the heart, and to distinguish 5-HT2B-mediated responses from those produced by 5-HT2A or 5-HT2C.

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.
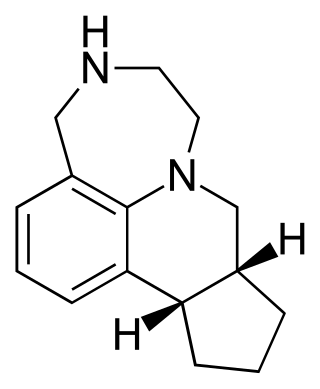
Vabicaserin was a novel antipsychotic and anorectic under development by Wyeth. As of 2010 it is no longer in clinical trials for the treatment of psychosis. It was also under investigation as an antidepressant but this indication appears to have been dropped as well.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor. Osemozotan has antidepressant, anxiolytic, antiobsessional, serenic, and analgesic effects in animal studies, and is used to investigate the role of 5-HT1A receptors in modulating the release of dopamine and serotonin in the brain, and their involvement in addiction to abused stimulants such as cocaine and methamphetamine.
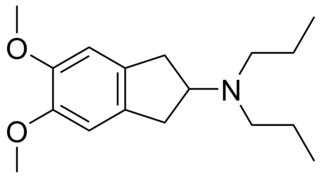
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.
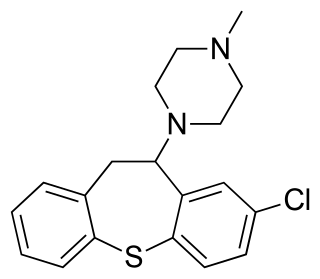
Clorotepine, also known as octoclothepin or octoclothepine, is an antipsychotic of the tricyclic group which was derived from perathiepin in 1965 and marketed in the Czech Republic by Spofa in or around 1971 for the treatment of schizophrenic psychosis.

SB-243213 is a research chemical which acts as a selective inverse agonist for the 5HT2C receptor and has anxiolytic effects. It has better than 100x selectivity for 5-HT2C over all other receptor subtypes tested, and a longer duration of action compared to older 5-HT2C antagonist ligands.




















