
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism.
cGMP-dependent protein kinase or protein kinase G (PKG) is a serine/threonine-specific protein kinase that is activated by cGMP. It phosphorylates a number of biologically important targets and is implicated in the regulation of smooth muscle relaxation, platelet function, sperm metabolism, cell division, and nucleic acid synthesis.
CAMK, also written as CaMK, is an abbreviation for the Ca2+/calmodulin-dependent protein kinase class of enzymes. CAMKs are activated by increases in the concentration of intracellular calcium ions (Ca2+) and calmodulin. When activated, the enzymes transfer phosphates from ATP to defined serine or threonine residues in other proteins, so they are serine/threonine-specific protein kinases. Activated CAMK is involved in the phosphorylation of transcription factors and therefore, in the regulation of expression of responding genes. CAMK also works to regulate the cell life cycle (i.e. programmed cell death), rearrangement of the cell's cytoskeletal network, and mechanisms involved in the learning and memory of an organism.

Telokin is an abundant protein found in smooth-muscle. It is identical to the C-terminus of myosin light-chain kinase. Telokin may play a role in the stabilization of unphosphorylated smooth-muscle myosin filaments. Because of its origin as the C-terminal end of smooth muscle myosin light chain kinase, it is called "telokin".
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II.

A serine/threonine protein kinase is a kinase enzyme that phosphorylates the OH group of serine or threonine. At least 125 of the 500+ human protein kinases are serine/threonine kinases (STK).

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can only synthesize 11 of the 20 standard amino acids, and in time of accelerated growth, histidine can be considered an essential amino acid.

Myosin light-chain phosphatase, more commonly called myosin phosphatase, is an enzyme that dephosphorylates the regulatory light chain of myosin II. This dephosphorylation reaction occurs in smooth muscle tissue and initiates the relaxation process of the muscle cells. Thus, myosin phosphatase undoes the muscle contraction process initiated by myosin light-chain kinase. The enzyme is composed of three subunits: the catalytic region, the myosin binding subunit (MYPT1), and a third subunit (M20) of unknown function. The catalytic region uses two manganese ions as catalysts to dephosphorylate the light-chains on myosin, which causes a conformational change in the myosin and relaxes the muscle. The enzyme is highly conserved and is found in all organisms’ smooth muscle tissue. While it is known that myosin phosphatase is regulated by rho-associated protein kinases, there is current debate about whether other molecules, such as arachidonic acid and cAMP, also regulate the enzyme.
A myosin light chain is a light chain of myosin. Myosin light chains were discovered by Chinese biochemist Cao Tianqin when he was a graduate student at the University of Cambridge in England.
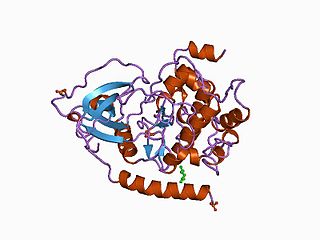
The protein kinase domain is a structurally conserved protein domain containing the catalytic function of protein kinases. Protein kinases are a group of enzymes that move a phosphate group onto proteins, in a process called phosphorylation. This functions as an on/off switch for many cellular processes, including metabolism, transcription, cell cycle progression, cytoskeletal rearrangement and cell movement, apoptosis, and differentiation. They also function in embryonic development, physiological responses, and in the nervous and immune system. Abnormal phosphorylation causes many human diseases, including cancer, and drugs that affect phosphorylation can treat those diseases.
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a polo kinase is a kinase enzyme i.e. one that catalyzes the chemical reaction

Serine/threonine-protein kinase Sgk3 is an enzyme that in humans is encoded by the SGK3 gene.

Serine/threonine-protein kinase MRCK alpha is an enzyme that in humans is encoded by the CDC42BPA gene.

Serine/threonine protein kinase WNK4 also known as WNK lysine deficient protein kinase 4 or WNK4, is an enzyme that in humans is encoded by the WNK4 gene. Missense mutations cause a genetic form of pseudohypoaldosteronism type 2, also called Gordon syndrome.

Serine/threonine-protein kinase Sgk2 is an enzyme that in humans is encoded by the SGK2 gene.

In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans. It is implicated in the development and progression of melanomas.

Myosin light chain kinase 2 also known as MYLK2 is an enzyme which in humans is encoded by the MYLK2 gene.
















