The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of neural integration in the central nervous system, and plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is the part of the brain responsible for cognition.

A motor neuron is a neuron whose cell body is located in the motor cortex, brainstem or the spinal cord, and whose axon (fiber) projects to the spinal cord or outside of the spinal cord to directly or indirectly control effector organs, mainly muscles and glands. There are two types of motor neuron – upper motor neurons and lower motor neurons. Axons from upper motor neurons synapse onto interneurons in the spinal cord and occasionally directly onto lower motor neurons. The axons from the lower motor neurons are efferent nerve fibers that carry signals from the spinal cord to the effectors. Types of lower motor neurons are alpha motor neurons, beta motor neurons, and gamma motor neurons.
The motor system is the set of central and peripheral structures in the nervous system that support motor functions, i.e. movement. Peripheral structures may include skeletal muscles and neural connections with muscle tissues. Central structures include cerebral cortex, brainstem, spinal cord, pyramidal system including the upper motor neurons, extrapyramidal system, cerebellum, and the lower motor neurons in the brainstem and the spinal cord.

In neuroanatomy, the trigeminal nerve (lit. triplet nerve), also known as the fifth cranial nerve, cranial nerve V, or simply CN V, is a cranial nerve responsible for sensation in the face and motor functions such as biting and chewing; it is the most complex of the cranial nerves. Its name (trigeminal, from Latin tri- 'three' and -geminus 'twin') derives from each of the two nerves (one on each side of the pons) having three major branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). The ophthalmic and maxillary nerves are purely sensory, whereas the mandibular nerve supplies motor as well as sensory (or "cutaneous") functions. Adding to the complexity of this nerve is that autonomic nerve fibers as well as special sensory fibers (taste) are contained within it.

The somatic nervous system (SNS), also known as voluntary nervous system, is a part of the peripheral nervous system (PNS) that links brain and spinal cord to skeletal muscles under conscious control, as well as to sensory receptors in the skin. The other part complementary to the somatic nervous system is the autonomic nervous system (ANS).

The pyramidal tracts include both the corticobulbar tract and the corticospinal tract. These are aggregations of efferent nerve fibers from the upper motor neurons that travel from the cerebral cortex and terminate either in the brainstem (corticobulbar) or spinal cord (corticospinal) and are involved in the control of motor functions of the body.

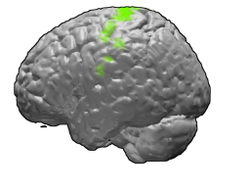
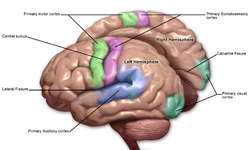
The motor cortex is the region of the cerebral cortex involved in the planning, control, and execution of voluntary movements. The motor cortex is an area of the frontal lobe located in the posterior precentral gyrus immediately anterior to the central sulcus.

Upper motor neurons (UMNs) is a term introduced by William Gowers in 1886. They are found in the cerebral cortex and brainstem and carry information down to activate interneurons and lower motor neurons, which in turn directly signal muscles to contract or relax. UMNs represent the major origin point for voluntary somatic movement.

The precentral gyrus is a prominent gyrus on the surface of the posterior frontal lobe of the brain. It is the site of the primary motor cortex that in humans is cytoarchitecturally defined as Brodmann area 4.

Betz cells are giant pyramidal cells (neurons) located within the fifth layer of the grey matter in the primary motor cortex. These neurons are the largest in the central nervous system, sometimes reaching 100 μm in diameter.

The premotor cortex is an area of the motor cortex lying within the frontal lobe of the brain just anterior to the primary motor cortex. It occupies part of Brodmann's area 6. It has been studied mainly in primates, including monkeys and humans. The functions of the premotor cortex are diverse and not fully understood. It projects directly to the spinal cord and therefore may play a role in the direct control of behavior, with a relative emphasis on the trunk muscles of the body. It may also play a role in planning movement, in the spatial guidance of movement, in the sensory guidance of movement, in understanding the actions of others, and in using abstract rules to perform specific tasks. Different subregions of the premotor cortex have different properties and presumably emphasize different functions. Nerve signals generated in the premotor cortex cause much more complex patterns of movement than the discrete patterns generated in the primary motor cortex.

The supplementary motor area (SMA) is a part of the motor cortex of primates that contributes to the control of movement. It is located on the midline surface of the hemisphere just in front of the primary motor cortex leg representation. In monkeys, the SMA contains a rough map of the body. In humans, the body map is not apparent. Neurons in the SMA project directly to the spinal cord and may play a role in the direct control of movement. Possible functions attributed to the SMA include the postural stabilization of the body, the coordination of both sides of the body such as during bimanual action, the control of movements that are internally generated rather than triggered by sensory events, and the control of sequences of movements. All of these proposed functions remain hypotheses. The precise role or roles of the SMA is not yet known.
In neuroanatomy, topographic map is the ordered projection of a sensory surface or an effector system to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.
Premovement neuronal activity in neurophysiological literature refers to neuronal modulations that alter the rate at which neurons fire before a subject produces movement. Through experimentation with multiple animals, predominantly monkeys, it has been shown that several regions of the brain are particularly active and involved in initiation and preparation of movement. Two specific membrane potentials, the bereitschaftspotential, or the BP, and contingent negative variation, or the CNV, play a pivotal role in premovement neuronal activity. Both have been shown to be directly involved in planning and initiating movement. Multiple factors are involved with premovement neuronal activity including motor preparation, inhibition of motor response, programming of the target of movement, closed-looped and open-looped tasks, instructed delay periods, short-lead and long-lead changes, and mirror motor neurons.
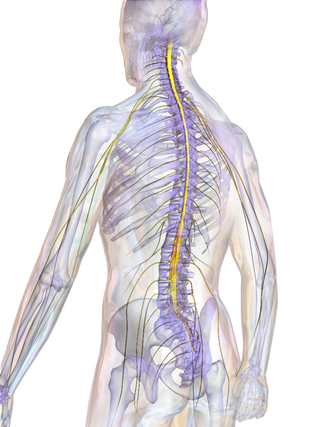
The spinal cord is a long, thin, tubular structure made up of nervous tissue that extends from the medulla oblongata in the lower brainstem to the lumbar region of the vertebral column (backbone) of vertebrate animals. The center of the spinal cord is hollow and contains a structure called the central canal, which contains cerebrospinal fluid. The spinal cord is also covered by meninges and enclosed by the neural arches. Together, the brain and spinal cord make up the central nervous system.

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.
Michael Steven Anthony Graziano is an American scientist and novelist who is currently a professor of Psychology and Neuroscience at Princeton University. His scientific research focuses on the brain basis of awareness. He has proposed the "attention schema" theory, an explanation of how, and for what adaptive advantage, brains attribute the property of awareness to themselves. His previous work focused on how the cerebral cortex monitors the space around the body and controls movement within that space. Notably he has suggested that the classical map of the body in motor cortex, the homunculus, is not correct and is better described as a map of complex actions that make up the behavioral repertoire. His publications on this topic have had a widespread impact among neuroscientists but have also generated controversy. His novels rely partly on his background in psychology and are known for surrealism or magic realism. Graziano also composes music including symphonies and string quartets.
Nonprimary motor cortex is a functionally defined portion of the frontal lobe. It includes two subdivisions, the premotor cortex and the supplementary motor cortex. Largely coincident with the cytoarchitecturally defined area 6 of Brodmann (human), it is located primarily in the rostral portion of the precentral gyrus and caudal portions of the superior frontal gyrus and the middle frontal gyrus, It aids in cerebral control of movement. Anatomically speaking, several nonprimary areas exist, and make direct connections with the spinal cord.

The corticospinal tract is a white matter motor pathway starting at the cerebral cortex that terminates on lower motor neurons and interneurons in the spinal cord, controlling movements of the limbs and trunk. There are more than one million neurons in the corticospinal tract, and they become myelinated usually in the first two years of life.

Eberhard Erich Fetz is an American neuroscientist, academic and researcher. He is a Professor of Physiology and Biophysics and DXARTS at the University of Washington.