A chordate is a deuterostomic animal belonging to the phylum Chordata. All chordates possess, at some point during their larval or adult stages, five distinctive physical characteristics (synapomorphies) that distinguish them from other taxa. These five synapomorphies are a notochord, a hollow dorsal nerve cord, an endostyle or thyroid, pharyngeal slits, and a post-anal tail. The name "chordate" comes from the first of these synapomorphies, the notochord, which plays a significant role in chordate body plan structuring and movements. Chordates are also bilaterally symmetric, have a coelom, possess a closed circulatory system, and exhibit metameric segmentation.
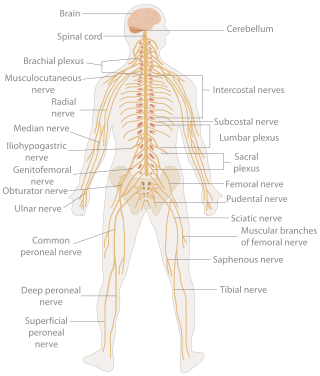
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes that impact the body, then works in tandem with the endocrine system to respond to such events. Nervous tissue first arose in wormlike organisms about 550 to 600 million years ago. In vertebrates, it consists of two main parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord. The PNS consists mainly of nerves, which are enclosed bundles of the long fibers, or axons, that connect the CNS to every other part of the body. Nerves that transmit signals from the brain are called motor nerves or efferent nerves, while those nerves that transmit information from the body to the CNS are called sensory nerves or afferent. Spinal nerves are mixed nerves that serve both functions. The PNS is divided into three separate subsystems, the somatic, autonomic, and enteric nervous systems. Somatic nerves mediate voluntary movement. The autonomic nervous system is further subdivided into the sympathetic and the parasympathetic nervous systems. The sympathetic nervous system is activated in cases of emergencies to mobilize energy, while the parasympathetic nervous system is activated when organisms are in a relaxed state. The enteric nervous system functions to control the gastrointestinal system. Both autonomic and enteric nervous systems function involuntarily. Nerves that exit from the cranium are called cranial nerves while those exiting from the spinal cord are called spinal nerves.

Hemichordata is a phylum which consists of triploblastic, enterocoelomate, and bilaterally symmetrical marine deuterostome animals, generally considered the sister group of the echinoderms. They appear in the Lower or Middle Cambrian and include two main classes: Enteropneusta, and Pterobranchia. A third class, Planctosphaeroidea, is known only from the larva of a single species, Planctosphaera pelagica. The class Graptolithina, formerly considered extinct, is now placed within the pterobranchs, represented by a single living genus Rhabdopleura.
Vetulicolia is a phylum of animals encompassing several extinct species belonging to the Cambrian Period. The phylum was created by Degan Shu and his research team in 2001, and named after Vetulicola cuneata, the first species of the phylum described in 1987. The vetulicolian body comprises two parts: a voluminous anterior forebody, tipped with an anteriorly positioned mouth and lined with a row of five round to oval-shaped features on each lateral side, which have been interpreted as gills ; and a posterior section that primitively comprises seven segments and functions as a tail. All vetulicolians lack preserved appendages of any kind, having no legs, feelers or even eyes. The area where the anterior and posterior parts join is constricted.

Bilateria is a large clade or infrakingdom of animals called bilaterians, characterized by bilateral symmetry during embryonic development. This means their body plans are laid around a longitudinal axis with a front and a rear end, as well as a left–right–symmetrical belly (ventral) and back (dorsal) surface. Nearly all bilaterians maintain a bilaterally symmetrical body as adults; the most notable exception is the echinoderms, which achieve secondary pentaradial symmetry as adults, but are bilaterally symmetrical as an embryo. Cephalization is also a characteristic feature among most bilaterians, where the special sense organs and central nerve ganglia become concentrated at the front/rostral end.

In the developing chordate, the neural tube is the embryonic precursor to the central nervous system, which is made up of the brain and spinal cord. The neural groove gradually deepens as the neural fold become elevated, and ultimately the folds meet and coalesce in the middle line and convert the groove into the closed neural tube. In humans, neural tube closure usually occurs by the fourth week of pregnancy.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

In zoology and developmental anatomy, the notochord is an elastic, rod-like anatomical structure found in many deuterostomal animals. A notochord is one of five synapomorphies used to define a species as a chordate.

Pikaia gracilens is an extinct, primitive chordate animal known from the Middle Cambrian Burgess Shale of British Columbia. Described in 1911 by Charles Doolittle Walcott as an annelid, and in 1979 by Harry B. Whittington and Simon Conway Morris as a chordate, it became "one of the most famous early chordate fossils," or "famously known as the earliest described Cambrian chordate". It is estimated to have lived during the latter period of the Cambrian explosion. Since its initial discovery, more than a hundred specimens have been recovered.

The lancelets, also known as amphioxi, consist of some 30 to 35 species of "fish-like" benthic filter feeding chordates in the subphylum Cephalochordata, class Leptocardii, and family Branchiostomatidae.
The acorn worms or Enteropneusta are a hemichordate class of invertebrates consisting of one order of the same name. The closest non-hemichordate relatives of the Enteropneusta are the echinoderms. There are 111 known species of acorn worm in the world, the main species for research being Saccoglossus kowalevskii. Two families—Harrimaniidae and Ptychoderidae—separated at least 370 million years ago.

Pharyngeal slits are filter-feeding organs found among deuterostomes. Pharyngeal slits are repeated openings that appear along the pharynx caudal to the mouth. With this position, they allow for the movement of water in the mouth and out the pharyngeal slits. It is postulated that this is how pharyngeal slits first assisted in filter-feeding, and later, with the addition of gills along their walls, aided in respiration of aquatic chordates. These repeated segments are controlled by similar developmental mechanisms. Some hemichordate species can have as many as 200 gill slits. Pharyngeal clefts resembling gill slits are transiently present during the embryonic stages of tetrapod development. The presence of pharyngeal arches and clefts in the neck of the developing human embryo famously led Ernst Haeckel to postulate that "ontogeny recapitulates phylogeny"; this hypothesis, while false, contains elements of truth, as explored by Stephen Jay Gould in Ontogeny and Phylogeny. However, it is now accepted that it is the vertebrate pharyngeal pouches and not the neck slits that are homologous to the pharyngeal slits of invertebrate chordates. Pharyngeal arches, pouches, and clefts are, at some stage of life, found in all chordates. One theory of their origin is the fusion of nephridia which opened both on the outside and the gut, creating openings between the gut and the environment.
The dorsal nerve cord is an anatomical feature found in chordate animals, mainly in the subphylum Vertebrata. It is one of the five embryonic features unique to all chordates, the other four being a notochord, a post-anal tail, an endostyle, and pharyngeal slits.

The ventral nerve cord is a major structure of the invertebrate central nervous system. It is the functional equivalent of the vertebrate spinal cord. The ventral nerve cord coordinates neural signaling from the brain to the body and vice versa, integrating sensory input and locomotor output. Because arthropods have an open circulatory system, decapitated insects can still walk, groom, and mate—illustrating that the circuitry of the ventral nerve cord is sufficient to perform complex motor programs without brain input.

Cerberus is a protein that in humans is encoded by the CER1 gene. Cerberus is a signaling molecule which contributes to the formation of the head, heart and left-right asymmetry of internal organs. This gene varies slightly from species to species but its overall functions seem to be similar.

Deuterostomes are bilaterian animals of the superphylum Deuterostomia, typically characterized by their anus forming before the mouth during embryonic development. Deuterostomia is further divided into 4 phyla; Chordata, Echinodermata, Hemichordata, the extinct Vetulicolia known from Cambrian fossils. The extinct clade Cambroernida is also though to be a member of Deuterostomia.
The evolution of nervous systems dates back to the first development of nervous systems in animals. Neurons developed as specialized electrical signaling cells in multicellular animals, adapting the mechanism of action potentials present in motile single-celled and colonial eukaryotes. Primitive systems, like those found in protists, use chemical signalling for movement and sensitivity; data suggests these were precursors to modern neural cell types and their synapses. When some animals started living a mobile lifestyle and eating larger food particles externally, they developed ciliated epithelia, contractile muscles and coordinating & sensitive neurons for it in their outer layer.
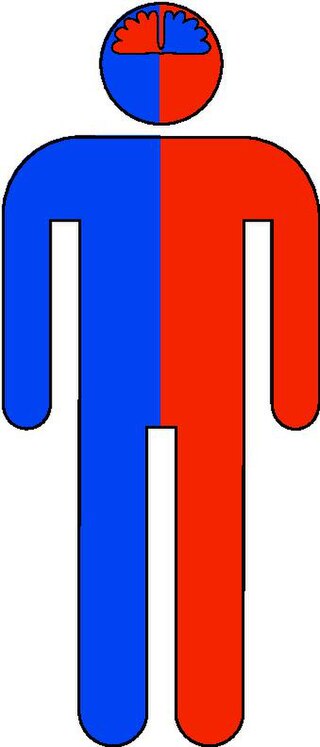
The contralateral organization of the forebrain is the property that the hemispheres of the cerebrum and the thalamus represent mainly the contralateral side of the body. Consequently, the left side of the forebrain mostly represents the right side of the body, and the right side of the brain primarily represents the left side of the body. The contralateral organization involves both executive and sensory functions. The contralateral organization is only present in vertebrates.
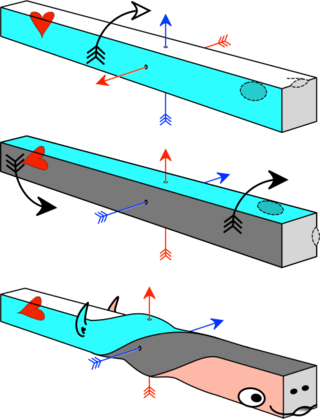
The axial twist theory is a scientific theory put forward to explain a range of unusual aspects of the body plan of vertebrates. It proposes that the rostral part of the head is "turned around" regarding the rest of the body. This end-part consists of the face as well as part of the brain. According to the theory, the vertebrate body has a left-handed chirality.
Left-right asymmetry is the process in early embryonic development that breaks the normal symmetry in the bilateral embryo. In vertebrates, left-right asymmetry is established early in development at a structure called the left-right organizer and leads to activation of different signalling pathways on the left and right of the embryo. This in turn cause several organs in adults to develop LR asymmetry, such as the tilt of the heart, the different number lung lobes on each side of the body and the position of the stomach and spleen on the right side of the body. If this process does not occur correctly in humans it can result in the syndromes heterotaxy or situs inversus.