A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of the cell and another. Biological membranes, in the form of eukaryotic cell membranes, consist of a phospholipid bilayer with embedded, integral and peripheral proteins used in communication and transportation of chemicals and ions. The bulk of lipids in a cell membrane provides a fluid matrix for proteins to rotate and laterally diffuse for physiological functioning. Proteins are adapted to high membrane fluidity environment of the lipid bilayer with the presence of an annular lipid shell, consisting of lipid molecules bound tightly to the surface of integral membrane proteins. The cell membranes are different from the isolating tissues formed by layers of cells, such as mucous membranes, basement membranes, and serous membranes.

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

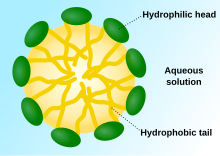
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue. Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine.

The RNA world is a hypothetical stage in the evolutionary history of life on Earth, in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. The term also refers to the hypothesis that posits the existence of this stage.

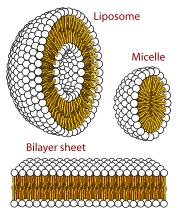
In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.

A hot spring, hydrothermal spring, or geothermal spring is a spring produced by the emergence of geothermally heated groundwater onto the surface of the Earth. The groundwater is heated either by shallow bodies of magma or by circulation through faults to hot rock deep in the Earth's crust. In either case, the ultimate source of the heat is the radioactive decay of naturally occurring radioactive elements in the Earth's mantle, the layer beneath the crust.

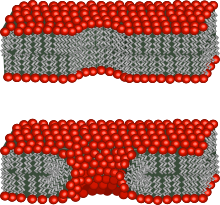
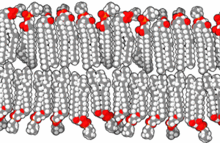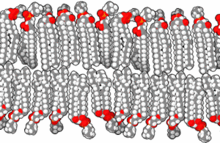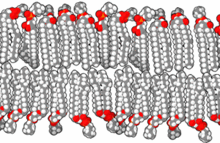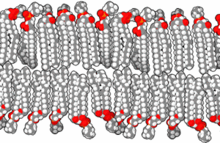
The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.
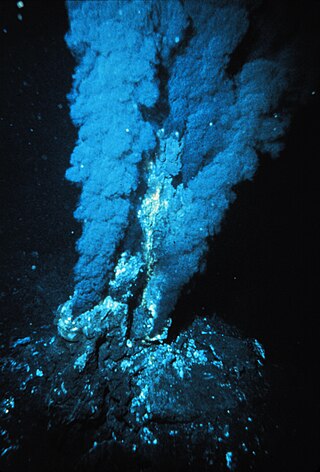
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspots. Hydrothermal deposits are rocks and mineral ore deposits formed by the action of hydrothermal vents.
The iron–sulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry, who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The hypothesis proposes that early life may have formed on the surface of iron sulfide minerals, hence the name. It was developed by retrodiction from extant biochemistry in conjunction with chemical experiments.
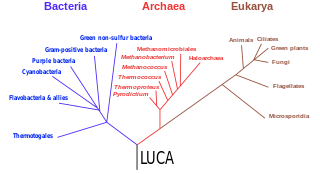
The last universal common ancestor (LUCA) is the hypothesized common ancestral cell from which the three domains of life, the Bacteria, the Archaea, and the Eukarya originated. It is suggested to have been a "cellular organism that had a lipid bilayer and used DNA, RNA, and protein". The LUCA has also been defined as "a hypothetical organism ancestral to all three domains". The LUCA is the point or stage at which the three domains of life diverged from preexisting forms of life. The nature of this point or stage of divergence remains a topic of research.
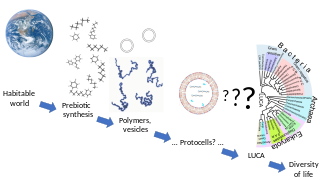
In biology, abiogenesis or the origin of life is the natural process by which life has arisen from non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to living entities on Earth was not a single event, but a process of increasing complexity involving the formation of a habitable planet, the prebiotic synthesis of organic molecules, molecular self-replication, self-assembly, autocatalysis, and the emergence of cell membranes. Many proposals have been made for different stages of the process, but the transition from non-life to life has never been observed experimentally.
Obcells are hypothetical proto-organisms or the earliest form of life. The term was first proposed by Thomas Cavalier-Smith in 2001. According to Cavalier-Smith's theory for the origin of the first cell, two cup-shaped obcells or hemicells fused to make a protocell with double-lipid layer envelope, internal genome and ribosomes, protocytosol, and periplasm.

The term chemoton refers to an abstract model for the fundamental unit of life introduced by Hungarian theoretical biologist Tibor Gánti. Gánti conceived the basic idea in 1952 and formulated the concept in 1971 in his book The Principles of Life. He suggested that the chemoton was the original ancestor of all organisms.
Evolution of cells refers to the evolutionary origin and subsequent evolutionary development of cells. Cells first emerged at least 3.8 billion years ago approximately 750 million years after Earth was formed.
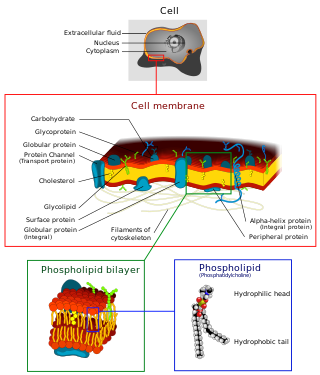
The cell membrane is a biological membrane that separates and protects the interior of a cell from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of a cell, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity, and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.
Jeewanu are synthetic chemical particles that possess cell-like structure and seem to have some functional properties; that is, they are a model of primitive cells, or protocells. It was first synthesised by Krishna Bahadur, an Indian chemist and his team in 1963. Using photochemical reaction, they produced coacervates, microscopic cell-like spheres from a mixture of simple organic and inorganic compounds. Bahadur named these particles 'Jeewanu' because they exhibit some of the basic properties of a cell, such as the presence of semipermeable membrane, amino acids, phospholipids and carbohydrates. Further, like living cells, they had several catalytic activities. Jeewanu are cited as models of protocells for the origin of life, and as artificial cells.
A scenario is a set of related concepts pertinent to the origin of life (abiogenesis), such as the iron-sulfur world. Many alternative abiogenesis scenarios have been proposed by scientists in a variety of fields from the 1950s onwards in an attempt to explain how the complex mechanisms of life could have come into existence. These include hypothesized ancient environments that might have been favourable for the origin of life, and possible biochemical mechanisms.
A proto-metabolism is a series of linked chemical reactions in a prebiotic environment that preceded and eventually turned into modern metabolism. Combining ongoing research in astrobiology and prebiotic chemistry, work in this area focuses on reconstructing the connections between potential metabolic processes that may have occurred in early Earth conditions. Proto-metabolism is believed to be simpler than modern metabolism and the Last Universal Common Ancestor (LUCA), as simple organic molecules likely gave rise to more complex metabolic networks. Prebiotic chemists have demonstrated abiotic generation of many simple organic molecules including amino acids, fatty acids, simple sugars, and nucleobases. There are multiple scenarios bridging prebiotic chemistry to early metabolic networks that occurred before the origins of life, also known as abiogenesis. In addition, there are hypotheses made on the evolution of biochemical pathways including the metabolism-first hypothesis, which theorizes how reaction networks dissipate free energy from which genetic molecules and proto-cell membranes later emerge. To determine the composition of key early metabolic networks, scientists have also used top-down approaches to study LUCA and modern metabolism.
A warm little pond is a hypothetical terrestrial shallow water environment on early Earth under which the origin of life could have occurred. The term was originally coined by Charles Darwin in an 1871 letter to his friend Joseph Dalton Hooker. This idea is related to later work such as the Oparin-Haldane hypothesis and the Miller–Urey experiment, which respectively provided a hypothesis for life’s origin from a primordial soup of organics and a proof of concept for the mechanism by which biomolecules and their precursors may have formed.