Muscular dystrophies (MD) are a genetically and clinically heterogeneous group of rare neuromuscular diseases that cause progressive weakness and breakdown of skeletal muscles over time. The disorders differ as to which muscles are primarily affected, the degree of weakness, how fast they worsen, and when symptoms begin. Some types are also associated with problems in other organs.
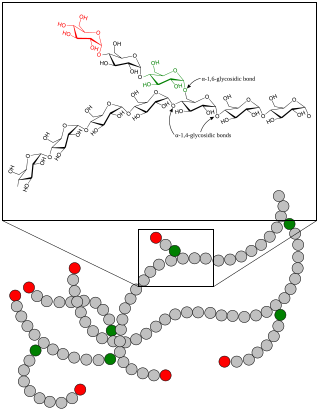
A glycogen storage disease is a metabolic disorder caused by a deficiency of an enzyme or transport protein affecting glycogen synthesis, glycogen breakdown, or glucose breakdown, typically in muscles and/or liver cells.

Limb–girdle muscular dystrophy (LGMD) is a genetically heterogeneous group of rare muscular dystrophies that share a set of clinical characteristics. It is characterised by progressive muscle wasting which affects predominantly hip and shoulder muscles. LGMD usually has an autosomal pattern of inheritance. It currently has no known cure or treatment.
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa

Duchenne muscular dystrophy (DMD) is a severe type of muscular dystrophy predominantly affecting boys. The onset of muscle weakness typically begins around age four, with rapid progression. Initially, muscle loss occurs in the thighs and pelvis, extending to the arms, which can lead to difficulties in standing up. By the age of 12, most individuals with Duchenne muscular dystrophy are unable to walk. Affected muscles may appear larger due to an increase in fat content, and scoliosis is common. Some individuals may experience intellectual disability, and females carrying a single copy of the mutated gene may show mild symptoms.

Fukuyama congenital muscular dystrophy (FCMD) is a rare, autosomal recessive form of muscular dystrophy (weakness and breakdown of muscular tissue) mainly described in Japan but also identified in Turkish and Ashkenazi Jewish patients; fifteen cases were first described on 1960 by Dr. Yukio Fukuyama.
Sarepta Therapeutics, Inc. is a medical research and drug development company with corporate offices and research facilities in Cambridge, Massachusetts, United States. Incorporated in 1980 as AntiVirals, shortly before going public the company changed its name from AntiVirals to AVI BioPharma soon with stock symbol AVII and in July 2012 changed name from AVI BioPharma to Sarepta Therapeutics and SRPT respectively. As of 2023, the company has four approved drugs.

Mitochondrial myopathies are types of myopathies associated with mitochondrial disease. Adenosine triphosphate (ATP), the chemical used to provide energy for the cell, cannot be produced sufficiently by oxidative phosphorylation when the mitochondrion is either damaged or missing necessary enzymes or transport proteins. With ATP production deficient in mitochondria, there is an over-reliance on anaerobic glycolysis which leads to lactic acidosis either at rest or exercise-induced.

Congenital muscular dystrophies are autosomal recessively-inherited muscle diseases. They are a group of heterogeneous disorders characterized by muscle weakness which is present at birth and the different changes on muscle biopsy that ranges from myopathic to overtly dystrophic due to the age at which the biopsy takes place.
The dystrophin-associated protein complex, also known as the dystrophin-associated glycoprotein complex is a multiprotein complex that includes dystrophin and the dystrophin-associated proteins. It is one of the two protein complexes that make up the costamere in striated muscle cells. The other complex is the integrin-vinculin-talin complex.

Sarcospan is a protein that in humans is encoded by the SSPN gene.
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
Drisapersen is an experimental drug that was under development by BioMarin, after acquisition of Prosensa, for the treatment of Duchenne muscular dystrophy. The drug is a 2'-O-methyl phosphorothioate oligonucleotide that alters the splicing of the dystrophin RNA transcript, eliminating exon 51 from the mature dystrophin mRNA.

Eteplirsen is a medication to treat, but not cure, some types of Duchenne muscular dystrophy (DMD), caused by a specific mutation. Eteplirsen only targets specific mutations and can be used to treat about 14% of DMD cases. Eteplirsen is a form of antisense therapy.
The mdx mouse is a popular model for studying Duchenne muscular dystrophy (DMD). The mdx mouse has a point mutation in its DMD gene, changing the amino acid coding for a glutamine to STOP codon. This causes the muscle cells to produce a small, nonfunctional dystrophin protein. As a result, the mouse has a mild form of DMD where there is increased muscle damage and weakness.

Ezutromid is an orally administered small molecule utrophin modulator involved in a Phase 2 clinical trial produced by Summit Therapeutics for the treatment of Duchenne muscular dystrophy (DMD). DMD is a fatal x-linked recessive disease affecting approximately 1 in 5000 males and is a designated orphan disease by the FDA and European Medicines Agency. Approximately 1/3 of the children obtain DMD as a result of spontaneous mutation in the dystrophin gene and have no family history of the disease. Dystrophin is a vital component of mature muscle function, and therefore DMD patients have multifarious forms of defunct or deficient dystrophin proteins that all manifest symptomatically as muscle necrosis and eventually organ failure. Ezutromid is theorized to maintain utrophin, a protein functionally and structurally similar to dystrophin that precedes and is replaced by dystrophin during development. Utrophin and dystrophin are reciprocally expressed, and are found in different locations in a mature muscle cell. However, in dystrophin-deficient patients, utrophin was found to be upregulated and is theorized to replace dystrophin in order to maintain muscle fibers. Ezutromid is projected to have the potential to treat all patients suffering with DMD as it maintains the production of utrophin to counteract the lack of dystrophin to retard muscle degeneration. Both the FDA and European Medicines Agency has given ezutromid an orphan drug designation. The FDA Office of Orphan Products and Development offers an Orphan Drug Designation program (ODD) that allows drugs aimed to treat diseases that affect less than 200,000 people in the U.S. monetary incentives such as a period of market exclusivity, tax incentives, and expedited approval processes.
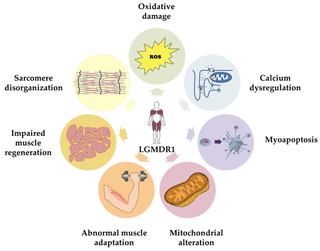
Calpainopathy is the most common type of autosomal recessive limb-girdle muscular dystrophy (LGMD). It preferentially affects the muscles of the hip girdle and shoulder girdle.
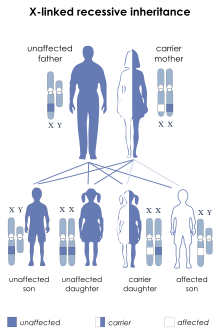
Dystrophinopathy refers to a spectrum of diseases due to mutations in the DMD gene, which encodes for the dystrophin protein found in muscle. The severe end of the spectrum includes Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and DMD-associated dilated cardiomyopathy. The mild end of the spectrum includes asymptomatic increases in serum creatine kinase and muscle cramps with myoglobinuria. Because dystrophin is located on the X chromosome, dystrophinopathy mainly affects males, whereas females range from being carriers, to having delayed-onset and mild disease, to having severe DMD.
Viltolarsen, sold under the brand name Viltepso, is a medication used for the treatment of Duchenne muscular dystrophy (DMD). Viltolarsen is a Morpholino antisense oligonucleotide.
Casimersen, sold under the brand name Amondys 45, is an antisense oligonucleotide medication used for the treatment of Duchenne muscular dystrophy (DMD) in people who have a confirmed mutation of the dystrophin gene that is amenable to exon 45 skipping. It is an antisense oligonucleotide of phosphorodiamidate morpholino oligomer (PMO). Duchenne muscular dystrophy is a rare disease that primarily affects boys. It is caused by low levels of a muscle protein called dystrophin. The lack of dystrophin causes progressive muscle weakness and premature death.