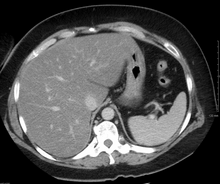
Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Tumor necrosis factor is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.

Alcoholic liver disease (ALD), also called alcohol-related liver disease (ARLD), is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.

Steatosis, also called fatty change, is abnormal retention of fat (lipids) within a cell or organ. Steatosis most often affects the liver – the primary organ of lipid metabolism – where the condition is commonly referred to as fatty liver disease. Steatosis can also occur in other organs, including the kidneys, heart, and muscle. When the term is not further specified, it is assumed to refer to the liver.

Alcoholic hepatitis is hepatitis due to excessive intake of alcohol. Patients typically have a history of at least 10 years of heavy alcohol intake, typically 8–10 drinks per day. It is usually found in association with fatty liver, an early stage of alcoholic liver disease, and may contribute to the progression of fibrosis, leading to cirrhosis. Symptoms may present acutely after a large amount of alcoholic intake in a short time period, or after years of excess alcohol intake. Signs and symptoms of alcoholic hepatitis include jaundice, ascites, fatigue and hepatic encephalopathy. Mild cases are self-limiting, but severe cases have a high risk of death. Severity in alcoholic hepatitis is determined several clinical prediction models such as the Maddrey's Discriminant Function and the MELD score.

Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.

Steatohepatitis is a type of fatty liver disease, characterized by inflammation of the liver with concurrent fat accumulation in liver. Mere deposition of fat in the liver is termed steatosis, and together these constitute fatty liver changes.

Metabolic dysfunction–associated steatotic liver disease (MASLD) is the name adopted in 2023 for the condition previously known as non-alcoholic fatty liver disease (NAFLD). This condition is diagnosed when there is excessive fat build-up in the liver, and at least one metabolic risk factor. When there is also moderate alcohol use, the term MetALD is used, and these are differentiated from alcoholic liver disease (ALD) when this is the sole cause of steatotic liver disease. The terms non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis have been used to describe different severities, the latter indicating the presence of further liver inflammation. NAFL is less dangerous than NASH and usually does not progress to it, but this progression may eventually lead to complications, such as cirrhosis, liver cancer, liver failure, and cardiovascular disease.
Acute fatty liver of pregnancy is a rare life-threatening complication of pregnancy that occurs in the third trimester or the immediate period after delivery. It is thought to be caused by a disordered metabolism of fatty acids by mitochondria in the fetus, caused by long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. This leads to decreased metabolism of long chain fatty acids by the feto-placental unit, causing subsequent rise in hepatotoxic fatty acids in maternal plasma. The condition was previously thought to be universally fatal, but aggressive treatment by stabilizing the mother with intravenous fluids and blood products in anticipation of early delivery has improved prognosis.

Phosphatidylethanolamine N-methyltransferase is a transferase enzyme which converts phosphatidylethanolamine (PE) to phosphatidylcholine (PC) in the liver. In humans it is encoded by the PEMT gene within the Smith–Magenis syndrome region on chromosome 17.

Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis due to damage caused by liver disease. Damage to the liver leads to repair of liver tissue and subsequent formation of scar tissue. Over time, scar tissue can replace normal functioning tissue, leading to the impaired liver function of cirrhosis. The disease typically develops slowly over months or years. Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and vomiting, and discomfort in the right upper quadrant of the abdomen. As the disease worsens, symptoms may include itchiness, swelling in the lower legs, fluid build-up in the abdomen, jaundice, bruising easily, and the development of spider-like blood vessels in the skin. The fluid build-up in the abdomen may develop into spontaneous infections. More serious complications include hepatic encephalopathy, bleeding from dilated veins in the esophagus, stomach, or intestines, and liver cancer.Stages of cirrhosis include compensated cirrhosis and decompensated cirrhosis.

Lipotoxicity is a metabolic syndrome that results from the accumulation of lipid intermediates in non-adipose tissue, leading to cellular dysfunction and death. The tissues normally affected include the kidneys, liver, heart and skeletal muscle. Lipotoxicity is believed to have a role in heart failure, obesity, and diabetes, and is estimated to affect approximately 25% of the adult American population.
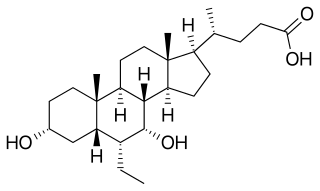
Obeticholic acid (OCA), sold under the brand name Ocaliva, is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It is used as a medication used to treat primary biliary cholangitis. Intercept Pharmaceuticals Inc. hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.
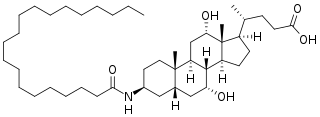
Aramchol is an investigational drug being developed by Galmed Pharmaceuticals as a first-in-class, potentially disease modifying treatment for nonalcoholic steatohepatitis, or NASH, a more advanced condition of non-alcoholic fatty liver disease.

Christos Socrates Mantzoros is a Greek American physician-scientist, practicing internist-endocrinologist, teacher and researcher. He is a professor of medicine at Harvard Medical School and an adjunct professor at Boston University School of Medicine. He currently serves as the chief of endocrinology, diabetes and metabolism at the VA Boston Healthcare System, where he created de novo a leading academic division true to its tripartite mission and as the founding director of human nutrition at Beth Israel Deaconess Medical Center (BIDMC), Harvard Medical School. Finally, he holds the editor-in-chief position of the journal Metabolism: Clinical and Experimental.

Melissa Palmer is an American hepatologist. She is recognized for her research and treatment of hepatitis and liver disease. Palmer is the Chief Medical Officer of Gannex Pharma, a wholly owned company of Ascletis Pharma.

Claudio Tiribelli is an Italian hepatologist best known for his studies on bilirubin and Kernicterus, a bilirubin-induced neurological condition.
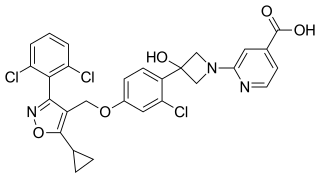
Cilofexor is a nonsteroidal farnesoid X receptor (FXR) agonist in clinical trials for the treatment of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and primary sclerosing cholangitis (PSC). It is being investigated for use alone or in combination with firsocostat, selonsertib, or semaglutide. In rat models and human clinical trials of NASH it has been shown to reduce fibrosis and steatosis, and in human clinical trials of PSC it improved cholestasis and reduced markers of liver injury.
Hepatokines are proteins produced by liver cells (hepatocytes) that are secreted into the circulation and function as hormones across the organism. Research is mostly focused on hepatokines that play a role in the regulation of metabolic diseases such as diabetes and fatty liver and include: Adropin, ANGPTL4, Fetuin-A, Fetuin-B, FGF-21, Hepassocin, LECT2, RBP4,Selenoprotein P, Sex hormone-binding globulin.
AXA1125 is an experimental drug developed by Axcella Health that "increased β-oxidation and improved bioenergetics in preclinical models". It was studied as a treatment for non-alcoholic fatty liver disease and long COVID.