
Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It may be sold in combination with paracetamol (acetaminophen) or as longer-acting formulations.
ATC code N02Analgesics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the WHO for the classification of drugs and other medical products. Subgroup N02 is part of the anatomical group N Nervous system.

Phenyltoloxamine is an antihistamine with sedative and analgesic effects. It is a member of the ethanolamine class of antihistaminergic agents and an anticholinergic.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Norway and Sweden and is used for severe pain.

Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to get reduced analgesic effects from tramadol. This also results in a ceiling effect which limits tramadol's range of therapeutic benefits to the treatment of moderate pain.

Oripavine is an opiate and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
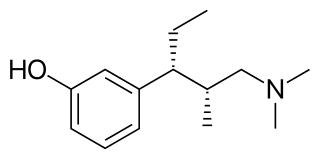
Tapentadol is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.
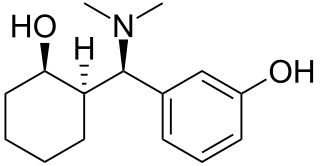
Ciramadol (WY-15,705) is an opioid analgesic that was developed in the late 1970s and is related to phencyclidine, tramadol, tapentadol and venlafaxine. It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential and a ceiling on respiratory depression which makes it a relatively safe drug. It has a slightly higher potency and effectiveness as an analgesic than codeine, but is weaker than morphine. Other side effects include sedation and nausea but these are generally less severe than with other similar drugs.

Etoxadrol (CL-1848C) is a dissociative anaesthetic drug that has been found to be an NMDA antagonist and produce similar effects to PCP in animals. Etoxadrol, along with another related drug dexoxadrol, were developed as analgesics for use in humans, but development was discontinued in the late 1970s after patients reported side effects such as nightmares and hallucinations.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.
The term seizure threshold is used to describe the balance between excitatory and inhibitory forces in the brain which affect how susceptible a person is to seizures. Those diagnosed with epilepsy or certain other neurological conditions are more vulnerable to seizures if the threshold is reduced, and should be compliant with their anticonvulsant drug regimen.
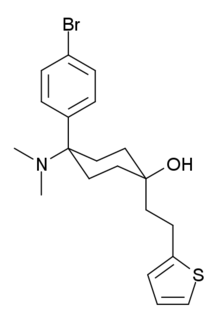
C-8813 (thiobromadol) is a potent μ-opioid receptor agonist with a distinctive chemical structure which is not closely related to other established families of opioid drugs. The trans-isomer was found to be around 591 times more potent than morphine in animal studies. The same study assigned a potency of 504 times that of morphine to the related compound BDPC.
"Pain ladder", or analgesic ladder, was created by the World Health Organization (WHO) as a guideline for the use of drugs in the management of pain. Originally published in 1986 for the management of cancer pain, it is now widely used by medical professionals for the management of all types of pain.
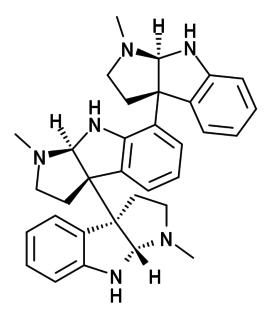
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family, as well as in the related species Calycodendron milnei.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others as well.

BDPC is a potent narcotic analgesic with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the strength of morphine in animal models. However, later studies assigned a value of 504 times the potency of morphine for the more active trans-isomer. To date, it is unknown if this drug has been used by humans, however, it was seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada.
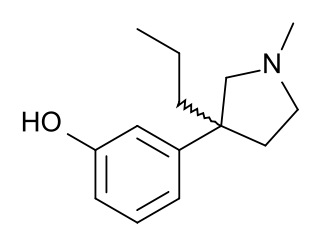
Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis. It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic effect is 1/50 of nalorphine.
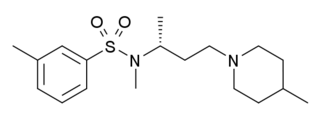
SB-258719 is a drug developed by GlaxoSmithKline which acts as a selective 5-HT7 receptor partial inverse agonist, and was the first such ligand identified for 5-HT7. Its use in research has mainly been in demonstrating the potential use for 5-HT7 agonists as potential novel analgesics, due to the ability of SB-258719 to block the analgesic effects of a variety of 5-HT7 agonists across several different testing models.

















