GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved G domain common to many GTPases.
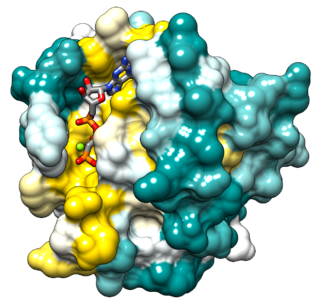
Ras is a family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in three-dimensional structure and regulate diverse cell behaviours.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.
GTPase-activating proteins or GTPase-accelerating proteins (GAPs) are a family of regulatory proteins whose members can bind to activated G proteins and stimulate their GTPase activity, with the result of terminating the signaling event. GAPs are also known as RGS protein, or RGS proteins, and these proteins are crucial in controlling the activity of G proteins. Regulation of G proteins is important because these proteins are involved in a variety of important cellular processes. The large G proteins, for example, are involved in transduction of signaling from the G protein-coupled receptor for a variety of signaling processes like hormonal signaling, and small G proteins are involved in processes like cellular trafficking and cell cycling. GAP's role in this function is to turn the G protein's activity off. In this sense, GAPs function is opposite to that of guanine nucleotide exchange factors (GEFs), which serve to enhance G protein signaling.

GTPase HRas also known as transforming protein p21 is an enzyme that in humans is encoded by the HRAS gene. The HRAS gene is located on the short (p) arm of chromosome 11 at position 15.5, from base pair 522,241 to base pair 525,549. HRas is a small G protein in the Ras subfamily of the Ras superfamily of small GTPases. Once bound to Guanosine triphosphate, H-Ras will activate a Raf kinase like c-Raf, the next step in the MAPK/ERK pathway.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

RAF proto-oncogene serine/threonine-protein kinase, also known as proto-oncogene c-RAF or simply c-Raf or even Raf-1, is an enzyme that in humans is encoded by the RAF1 gene. The c-Raf protein is part of the ERK1/2 pathway as a MAP kinase (MAP3K) that functions downstream of the Ras subfamily of membrane associated GTPases. C-Raf is a member of the Raf kinase family of serine/threonine-specific protein kinases, from the TKL (Tyrosine-kinase-like) group of kinases.

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

The KRAS gene provides instructions for making a protein called K-Ras, part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). The K-Ras protein is a GTPase, which means it converts a molecule called GTP into another molecule called GDP. In this way the K-Ras protein acts like a switch that is turned on and off by the GTP and GDP molecules. To transmit signals, it must be turned on by attaching (binding) to a molecule of GTP. The K-Ras protein is turned off (inactivated) when it converts the GTP to GDP. When the protein is bound to GDP, it does not relay signals to the cell's nucleus. It is called KRAS because it was first identified as an oncogene in KirstenRAt Sarcoma virus. The viral oncogene was derived from cellular genome. Thus, KRAS gene in cellular genome is called a proto-oncogene.
Son of sevenless homolog 1 is a protein that in humans is encoded by the SOS1 gene.

FYVE, RhoGEF and PH domain-containing protein 1 (FGD1) also known as faciogenital dysplasia 1 protein (FGDY), zinc finger FYVE domain-containing protein 3 (ZFYVE3), or Rho/Rac guanine nucleotide exchange factor FGD1 is a protein that in humans is encoded by the FGD1 gene that lies on the X chromosome. Orthologs of the FGD1 gene are found in dog, cow, mouse, rat, and zebrafish, and also budding yeast and C. elegans. It is a member of the FYVE, RhoGEF and PH domain containing family.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.
RHEB also known as Ras homolog enriched in brain (RHEB) is a GTP-binding protein that is ubiquitously expressed in humans and other mammals. The protein is largely involved in the mTOR pathway and the regulation of the cell cycle.

RasGEF domain is domain found in the CDC25 family of guanine nucleotide exchange factors for Ras-like small GTPases.

Rho guanine nucleotide exchange factor 6 is a protein that, in humans, is encoded by the ARHGEF6 gene.

RhoG is a small monomeric GTP-binding protein, and is an important component of many intracellular signalling pathways. It is a member of the Rac subfamily of the Rho family of small G proteins and is encoded by the gene RHOG.
Ras-related protein M-Ras, also known as muscle RAS oncogene homolog and R-Ras3, is a protein that in humans is encoded by the MRAS gene on chromosome 3. It is ubiquitously expressed in many tissues and cell types. This protein functions as a signal transducer for a wide variety of signaling pathways, including those promoting neural and bone formation as well as tumor growth. The MRAS gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

GRB2-related adapter protein is a protein that in humans is encoded by the GRAP gene.
Rap1 is a small GTPase, which are small cytosolic proteins that act like cellular switches and are vital for effective signal transduction. There are two isoforms of the Rap1 protein, each encoded by a separate gene, RAP1A and RAP1B. Rap1 belongs to Ras-related protein family.















