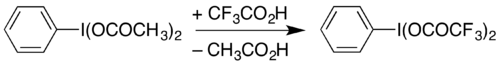| |
| Names | |
|---|---|
| Preferred IUPAC name Phenyl-λ3-iodanediyl diacetate | |
| Other names Bis(acetoxy)(phenyl)iodane Bis(acetato-O)phenyliodine Bis(acetoxy)iodobenzene (BAIB) (Diacetoxyiodo)benzene I,I-Diacetatoiodobenzene Iodobenzene diacetate Iodosobenzene I,I-diacetate Phenyliodine(III) diacetate (PIDA) Phenyliodo diacetate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.019.826 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H11IO4 | |
| Molar mass | 322.098 g·mol−1 |
| Appearance | white powder |
| Melting point | 163–165 °C (325–329 °F; 436–438 K) |
| reacts | |
| Solubility | soluble in acetic acid, acetonitrile, dichloromethane |
| Structure [1] [2] | |
| orthorhombic | |
| Pnn2 | |
| T-shaped molecular geometry | |
| Related compounds | |
Related compounds | (Bis(trifluoroacetoxy)iodo)benzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
(Diacetoxyiodo)benzene, also known as phenyliodine(III) diacetate (PIDA) is a hypervalent iodine chemical with the formula C
6H
5I(OCOCH
3)
2. It is used as an oxidizing agent in organic chemistry.