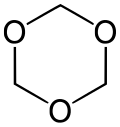
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,3,5-Trioxane | |||
| Other names s-Trioxane; 1,3,5-Trioxacyclohexane; Trioxymethylene; Metaformaldehyde | |||
| Identifiers | |||
3D model (JSmol) | |||
| 102769 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.466 | ||
| EC Number |
| ||
| 2230 | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1325 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H6O3 | |||
| Molar mass | 90.078 g·mol−1 | ||
| Appearance | White crystalline solid | ||
| Density | 1.17 g/cm3 (65 °C) [1] | ||
| Melting point | 62 °C (144 °F; 335 K) [1] | ||
| Boiling point | 115 °C (239 °F; 388 K) [1] | ||
| 221 g/L [1] | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Warning | |||
| H228, H335, H361d | |||
| P201, P202, P210, P240, P241, P261, P271, P280, P281, P304+P340, P308+P313, P312, P370+P378, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 45 [1] °C (113 °F; 318 K) | ||
| Related compounds | |||
Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,3,5-Trioxane, sometimes also called trioxane, is a chemical compound with molecular formula C3H6O3. It is a white, highly water-soluble solid with a chloroform-like odor. It is a stable cyclic trimer of formaldehyde, and one of the three trioxane isomers; its molecular backbone consists of a six-membered ring with three carbon atoms alternating with three oxygen atoms.