
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is only derived from dryed biomass, In addition to ethanol, acetic acid, and sugar, furfural is one of the oldest organic chemicals available readily purified from natural precursors.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
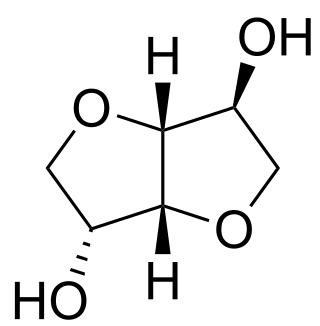
Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.

Hydroxymethylfurfural (HMF), also known as 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars. It is a white low-melting solid which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.

Furfuryl alcohol is an organic compound containing a furan substituted with a hydroxymethyl group. It is a colorless liquid, but aged samples appear amber. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstable in water. It is soluble in common organic solvents.

2,5-Dimethylfuran is a heterocyclic compound with the formula (CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.
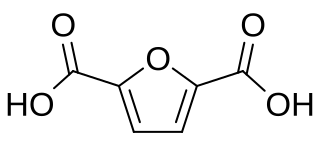
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.

Methoxymethylfurfural is an organic compound derived from dehydration of sugars and subsequent etherification with methanol. This colorless liquid is soluble in a wide range of solvents including lower alcohols. The molecule is a derivative of furan, containing both aldehyde and ether (methoxymethyl) functional groups. MMF has been detected in the leaves and roots of Chilean Jaborosa magellanica (Solanaceae). It has a typical odor suggestive of maraschino cherries. MMF can be made from a wide range of carbohydrate containing feedstocks including sugar, starch and cellulose using a chemical catalytic process and is a potential "carbon-neutral" feedstock for fuels and chemicals.

Dimethylol ethyleneurea is an organic compound derived from formaldehyde and urea. It is a colourless solid that is used for treating cellulose-based heavy fabrics to inhibit wrinkle formation. Dimethylol ethylene urea (DMEU) bonds with the hydroxyl groups present in long cellulose chains and prevents the formation hydrogen bonding between the chains, the primary cause of wrinkling. This treatment produces permanently wrinkle-resistant fabrics and is different from the effects achieved from using fabric softeners. An additional names for DMEU includes 1,3-bis(hydroxymethyl)-tetrahydro-2-imidazolone.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.

Levoglucosenone is an organic compound with the formula [OCH2(CH)4CO2]. A pale yellow liquid, it is an unsaturated bicyclic ketone-diether formed from levoglucosan by loss of two molecules of water. As a product of the acid-catalysed pyrolysis of cellulose, D-glucose, and levoglucosan, this liquid hydrocarbon is of interest as a biofuel and biofeedstock.

Tellurophenes are the tellurium analogue of thiophenes and selenophenes.

2,5-Bis(hydroxymethyl)pyrrole is an organic chemical compound with formula C6H9O2N, or (HOCH2)2(C4H3N). Its molecule can be described as that of pyrrole C4H5N with hydroxymethyl groups HO−CH2− replacing the two hydrogen atoms adjacent to the nitrogen atom.
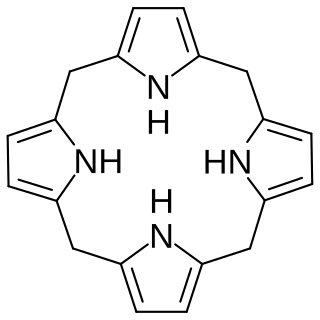
Hexahydroporphine is an organic chemical compound with formula C20H20N4. The molecule consists of four pyrrole rings connected by methylene bridges −CH2− into a larger (non-aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one. As indicated by the name, it may be viewed as derived from porphine by the addition of six hydrogen atoms: four on the methine bridges, and two on the nitrogen atoms.
1,2,6-Hexanetriol is a trivalent alcohol with two primary and one secondary hydroxy group. It is similar to glycerol in many respects and is used as a substitute for glycerol in many applications due to its more advantageous properties, such as higher thermal stability and lower hygroscopicity.

2,5-Furandicarboxaldehyde (FDC) is an organic compound with the molecular formula C4H2O(CHO)2. It consists of a furan ring with aldehyde groups on the 2 and 5 position. It is therefore classified as a dialdehyde.

















