Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber; he correctly deduced the empirical formula C5H8.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
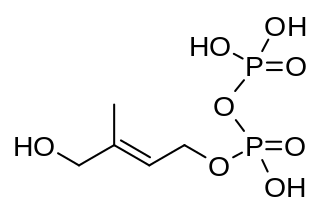
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis. The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.

DXP reductoisomerase is an enzyme that interconverts 1-deoxy-D-xylulose 5-phosphate (DXP) and 2-C-methyl-D-erythritol 4-phosphate (MEP).

2-C-Methyl-D-erythritol 4-phosphate (MEP) is an intermediate on the MEP pathway of isoprenoid precursor biosynthesis. It is the first committed metabolite on that pathway on the route to IPP and DMAPP.
In enzymology, a 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase (HMB-PP synthase, IspG, EC 1.17.7.1) is an enzyme that catalyzes the chemical reaction

The enzyme aminocyclopropane-1-carboxylic acid synthase catalyzes the synthesis of 1-Aminocyclopropane-1-carboxylic acid (ACC), a precursor for ethylene, from S-Adenosyl methionine, an intermediate in the Yang cycle and activated methyl cycle and a useful molecule for methyl transfer:
2-C-Methyl-D-erythritol 2,4-cyclodiphosphate synthase is a zinc-dependent enzyme and a member of the YgbB N terminal protein domain, which participates in the MEP pathway of isoprenoid precursor biosynthesis. It catalyzes the following reaction:
The enzyme indole-3-glycerol-phosphate lyase catalyzes the chemical reaction

In biochemistry, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
In enzymology, a 1-deoxy-d-xylulose-5-phosphate synthase (EC 2.2.1.7) is an enzyme in the non-mevalonate pathway that catalyzes the chemical reaction
In enzymology, a digalactosyldiacylglycerol synthase is an enzyme that catalyzes the chemical reaction

In enzymology, a 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase is an enzyme that catalyzes the chemical reaction:
In enzymology, a 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol kinase is an enzyme that catalyzes the chemical reaction
4-Diphosphocytidyl-2-C-methylerythritol is an intermediate in the MEP pathway of isoprenoid precursor biosynthesis. It is produced by the enzyme 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase (IspD) and is a substrate for CDP-ME kinase (IspE).
4-Diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate is an intermediate in the MEP pathway of isoprenoid precursor biosynthesis.
4-Hydroxy-3-methylbut-2-enyl diphosphate reductase (EC 1.17.1.2, isopentenyl-diphosphate:NADP+ oxidoreductase, LytB, (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase, HMBPP reductase, IspH, LytB/IspH) is an enzyme in the non-mevalonate pathway. It acts upon (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (or "HMB-PP").

In molecular biology, YgbB is a protein domain. This entry makes reference to a number of proteins from eukaryotes and prokaryotes which share this common N-terminal signature and appear to be involved in terpenoid biosynthesis. The YgbB protein is a putative enzyme thought to aid terpenoid and isoprenoid biosynthesis, a vital chemical in all living organisms. This protein domain is part of an enzyme which catalyses a reaction in a complex pathway.
Michel Rohmer, born on 31 January 1948, is a French chemist specialising in the chemistry of micro-organisms. He has particularly studied isoprenoids.