
Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.
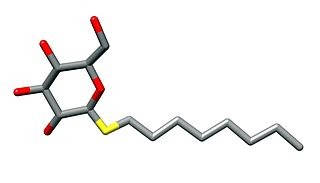
n-Octyl β-d-thioglucopyranoside is a mild nonionic detergent that is used for cell lysis or to solubilise membrane proteins without denaturing them. This is particularly of use in order to crystallise them or to reconstitute them into lipid bilayers. It has a critical micelle concentration of 9 mM.
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal. It was discovered by the carbohydrate chemist Robert J. Ferrier.

β-Glucosidase is an enzyme that catalyses the following reaction:

α-Glucosidase is a glucosidase located in the brush border of the small intestine that acts upon α(1→4) bonds:
The term glycosynthase refers to a class of proteins that have been engineered to catalyze the formation of a glycosidic bond. Glycosynthase are derived from glycosidase enzymes, which catalyze the hydrolysis of glycosidic bonds. They were traditionally formed from retaining glycosidase by mutating the active site nucleophilic amino acid to a small non-nucleophilic amino acid. More modern approaches use directed evolution to screen for amino acid substitutions that enhance glycosynthase activity.
The enzyme N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase (EC 3.1.4.45) catalyzes the reaction
The enzyme 6-phospho-β-glucosidase (EC 3.2.1.86) catalyzes the following reaction:
The enzyme amygdalin β-glucosidase (EC 3.2.1.117) catalyzes the following chemical reaction:
In enzymology, a beta-apiosyl-beta-glucosidase (EC 3.2.1.161) is an enzyme that catalyzes the chemical reaction
The enzyme coniferin β-glucosidase (EC 3.2.1.126) catalyzes the following chemical reaction:

In enzymology, a glucosylceramidase (EC 3.2.1.45) is an enzyme that catalyzes the chemical reaction
The enzyme maltose-6′-phosphate glucosidase (EC 3.2.1.122) catalyzes the following chemical reaction:
The enzyme protein-glucosylgalactosylhydroxylysine glucosidase (EC 3.2.1.107) catalyzes the following chemical reaction:
In enzymology, a prunasin β-glucosidase (EC 3.2.1.118) is an enzyme that catalyzes the chemical reaction
The enzyme steryl-β-glucosidase (EC 3.2.1.104) catalyzes the following chemical reaction:

Cytosolic beta-glucosidase, also known as cytosolic beta-glucosidase-like protein 1, is a beta-glucosidase enzyme that in humans is encoded by the GBA3 gene.
4-Hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl glucoside beta-D-glucosidase (EC 3.2.1.182, DIMBOAGlc hydrolase, DIMBOA glucosidase) is an enzyme with systematic name (2R)-4-hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl beta-D-glucopyranoside beta-D-glucosidase. This enzyme catalyses the following chemical reaction

1-Deoxynojirimycin, also called duvoglustat or moranolin, is an alpha-glucosidase inhibitor, most commonly found in mulberry leaves. Although it can be obtained in small quantities by brewing an herbal tea from mulberry leaves, interest in commercial production has led to research on developing mulberry tea higher in DNJ, and on alternate routes of production, such as via Bacillus species.

An iminosugar, also known as an iminosaccharide, is any analog of a sugar where a nitrogen atom has replaced the oxygen atom in the ring of the structure.








