
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed, usually as a base, in organic synthesis.

The R-21 was a submarine-launched ballistic missile in service with the Soviet Union between 1963 and 1989. It was the first Soviet nuclear missile that could be launched from a submerged submarine, and also had twice the range of earlier missiles. It replaced the R-11FM and R-13 (SS-N-4) on many Golf and Hotel-class submarines, and was in turn superseded by the R-27 missile carried by Yankee-class submarines.
Substances, mixtures and exposure circumstances in this list have been classified by the International Agency for Research on Cancer (IARC) as group 3: The agent is not classifiable as to its carcinogenicity to humans. This category is used most commonly for agents, mixtures and exposure circumstances for which the evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Ponceau 2R, Xylidine ponceau, Ponceau G, Red R, Acid Red 26, Food Red 5, or C.I. 16150 is a red azo dye used in histology for staining. It is easily soluble in water and slightly in ethanol. It usually comes as a disodium salt.

m-Xylene (meta-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The m- stands for meta-, indicating that the two methyl groups in m-xylene occupy positions 1 and 3 on a benzene ring. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and p-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable.
Ponceau is the generic name for a family of azo dyes, and may refer to:
Xylidine can refer to any of the six isomers of xylene amine, or any mixture of them.
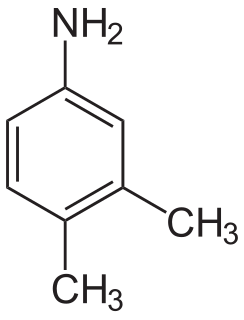
3,4-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless solid. It is a precursor for the production of riboflavin (vitamin B2).

2,6-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives are the anesthetics lidocaine, bupivacaine, mepivacaine, and etidocaine.

2,5-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives include Solvent Yellow 30, Solvent Red 22, Acid Red 65, and Solvent Red 26.
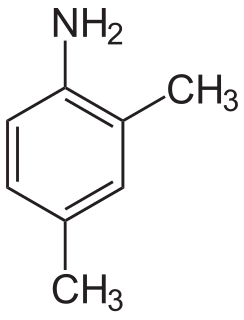
2,4-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives include the veterinary drug cymiazole and the colorant Pigment Yellow 81.
An antiknock agent is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, when used in high compression internal combustion engines, has a tendency to knock and/or to ignite early before the correctly timed spark occurs.

o-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.

m-Anisidine is an organic compound with the formula CH3OC6H4NH2. A clear light yellow or amber color liquid, commercial samples can appear brown owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.
p-Anisidine (para-anisidine) is an organic compound with the formula CH3OC6H4NH2. A white solid, commercial samples can appear grey-brown owing to air oxidation. It is one of three isomers of anisidine, methoxy-containing anilines. It is prepared by reduction of 4-nitroanisole.
The molecular formula C8H11N (molar mass: 121.18 g/mol) may refer to:
The BMW 109-558 is a liquid fuelled sustainer rocket motor developed by BMW at their Bruckmühl facility, in Germany during the Second World War.
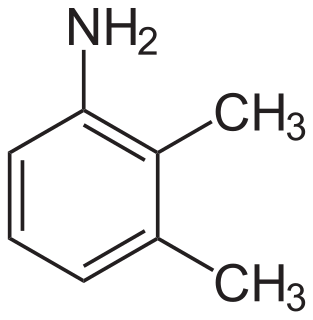
2,3-Xylidine is the organic compound with the formula C6H3(CH3)2NH2. it is one of several isomeric xylidines. It is a colorless viscous liquid. The compound is used in the production of the drug mefenamic acid and the herbicide xylachlor.

The SEPR 84 is a family of liquid-propellant rocket engines used as boosters for the Dassault Mirage III mixed-power high-altitude interceptor aircraft of the 1960s. The engine was one of several similar developed by SEPR.
















