
Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.

Quinoline Yellow SS is a bright yellow dye with green shade. It is insoluble in water, but soluble in nonpolar organic solvents. Quinoline yellow is representative of a large class of quinophthalone pigments. It is suggested that quinoline yellow exhibits excited-state intramolecular proton transfer (ESIPT) behavior and the behavior might be the cause of its decent photostability, by recent spectroscopic study.

3,3'-Dichlorobenzidine is an organic compound with the formula (C6H3Cl(NH2))2. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of diarylide yellow pigments used in the production of printing inks. Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity.

Phthalonitrile is an organic compound with the formula C6H4(CN)2, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers.

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs), a family of compounds with many uses.

Pigment Yellow 81 is an organic compound that is classified as a diarylide pigment. It is used as a yellow colorant.

Pigment Violet 29 is an organic compound that is used as a pigment and vat dye. Its colour is dark red purple, or bordeaux.
Pigment Red 190, also called Vat Red 29, is a synthetic organic compound that is used both as a pigment and as a vat dye. Although structurally a derivative of perylene, it is produced from acenaphthene.

Perylenetetracarboxylic dianhydride (PTCDA) is an organic dye molecule and an organic semiconductor. It is used as a precursor to a class of molecules known as Rylene dyes, which are useful as pigments and dyes. It is a dark red solid with low solubility in aromatic solvents. The compound has attracted much interest as an organic semiconductor.
Pigment Red 178 is an organic compound that is used as a pigment. Structurally, it is a derivative of perylene, although it is produced from perylenetetracarboxylic dianhydride by derivatization with 4-aminoazobenzene.

Pigment Red 179 is an organic compound that is used as a pigment. Structurally, it is a derivative of perylene, although it is produced from perylenetetracarboxylic dianhydride by derivatization with methylamine.
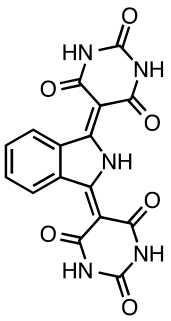
Pigment yellow 139 is an organic compound that is used as a yellow-orange pigment. It is classified as a derivative of isoindoline. This yellow-orange solid is virtually insoluble in most solvents.

Anthanthrone is a synthetic anthraquinone. Its derivative 4,10-dibromoanthanthrone is a component of some industrial paints. It is prepared from naphthostyril.
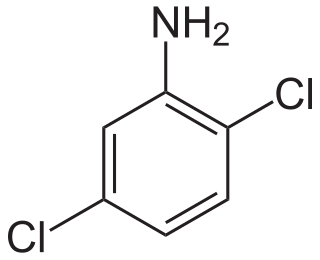
2,5-Dichloroaniline is an organic compound with the formula C6H3Cl2NH2. One of six isomers of dichloroaniline, it is a colorless solid that is insoluble in water. It is produced by hydrogenation of 1,4-dichloro-2-nitrobenzene. It is a precursor to dyes and pigments, e.g., Pigment Yellow 10.
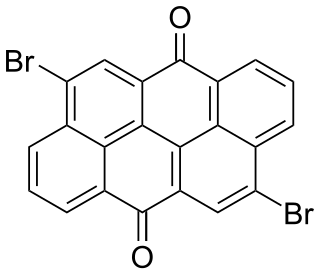
Dibromoanthanthrone is a scarlet or orange-red-hue synthetic organic colourant.

Pigment violet 23 is an organic compound that is a commercial pigment. It is member of the dioxazine family of heterocyclic compounds, but derived from carbazoles. It is prepared by condensation of chloranil and 3-amino-N-ethylcarbazole. It has a centrosymmetric angular structure. For many years, the structure was assigned, incorrectly, as having a "linear structure" which differ in terms of the carbazole ring fusion.
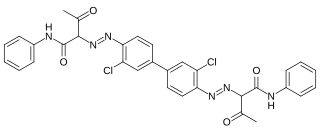
Pigment Yellow 12 is an organic compound and an azo compound. It is a widely used yellow pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Yellow 13, wherein the two phenyl groups are replaced by 2,4-xylyl.

3-Aminopentane is the organic compound with the formula (CH3CH2)2CHNH2. It is a colorless liquid. It is of interest for producing soluble imides and imines without introducing a chiral center.
















