 | |
 | |
| Names | |
|---|---|
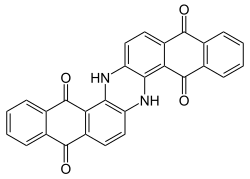
| Preferred IUPAC name 6,15-Dihydrodinaphtho[2,3-a:2′,3′-h]phenazine-5,9,14,18-tetrone | |
| Other names C.I. vat blue 4, carbon paper blue, blue O, carbanthrene blue 2R, fenan blue RSN, graphtol blue RL, medium blue, monolite fast blue 3R, indanthrene, indanthrone, pigment blue 60, C.I. 69800 | |
| Identifiers | |
3D model (JSmol) | |
| 367131 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.251 |
| E number | E130 (colours) |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C28H14N2O4 | |
| Molar mass | 442.430 g·mol−1 |
| Appearance | dark blue solid |
| Density | 1.6 g/ml |
| Melting point | 470-500 °C (decomposes) |
| Insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Indanthrone blue, also called indanthrene, is an organic compound with the formula (C14H6O2NH)2. It is a dark blue solid that is a common dye as well as a precursor to other dyes. [1]
