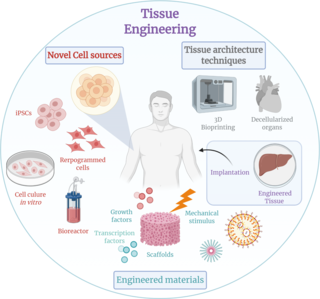
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can be considered as a field of its own.
Organ culture is the cultivation of either whole organs or parts of organs in vitro. It is a development from tissue culture methods of research, as the use of the actual in vitro organ itself allows for more accurate modelling of the functions of an organ in various states and conditions.

A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass. These cells are involved in:

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.
Cell culture or tissue culture is the process by which cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. They need to be kept at body temperature (37 °C) in an incubator. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or rich medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate to form an adherent culture as a monolayer (one single-cell thick), whereas others can be grown free floating in a medium as a suspension culture. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Tissue culture commonly refers to the culture of animal cells and tissues, with the more specific term plant tissue culture being used for plants. The lifespan of most cells is genetically determined, but some cell-culturing cells have been 'transformed' into immortal cells which will reproduce indefinitely if the optimal conditions are provided.
Matrigel is the trade name for the solubilized basement membrane matrix secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells produced by Corning Life Sciences. Matrigel resembles the laminin/collagen IV-rich basement membrane extracellular environment found in many tissues and is used by cell biologists as a substrate for culturing cells.
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. The name perlecan comes from its appearance as a "string of pearls" in rotary shadowed images.

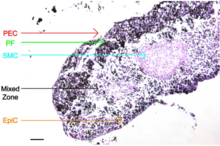
An organoid is a miniaturised and simplified version of an organ produced in vitro in three dimensions that mimics the key functional, structural, and biological complexity of that organ. It is derived from one or a few cells from a tissue, embryonic stem cells, or induced pluripotent stem cells, which can self-organize in three-dimensional culture owing to their self-renewal and differentiation capacities. The technique for growing organoids has rapidly improved since the early 2010s, and The Scientist named it one of the biggest scientific advancements of 2013. Scientists and engineers use organoids to study development and disease in the laboratory, for drug discovery and development in industry, personalized diagnostics and medicine, gene and cell therapies, tissue engineering, and regenerative medicine.
Magnetofection is a transfection method that uses magnetic fields to concentrate particles containing vectors to target cells in the body. Magnetofection has been adapted to a variety of vectors, including nucleic acids, non-viral transfection systems, and viruses. This method offers advantages such as high transfection efficiency and biocompatibility which are balanced with limitations.

T-cadherin, also known as cadherin 13, H-cadherin (heart), and CDH13, is a unique member of the cadherin protein family. Unlike typical cadherins that span across the cell membrane with distinct transmembrane and cytoplasmic domains, T-cadherin lacks these features and is instead anchored to the cell's plasma membrane through a GPI anchor.

Cadherin-2 also known as Neural cadherin (N-cadherin), is a protein that in humans is encoded by the CDH2 gene. CDH2 has also been designated as CD325 . Cadherin-2 is a transmembrane protein expressed in multiple tissues and functions to mediate cell–cell adhesion. In cardiac muscle, Cadherin-2 is an integral component in adherens junctions residing at intercalated discs, which function to mechanically and electrically couple adjacent cardiomyocytes. Alterations in expression and integrity of Cadherin-2 has been observed in various forms of disease, including human dilated cardiomyopathy. Variants in CDH2 have also been identified to cause a syndromic neurodevelopmental disorder.

Cadherin-5, or VE-cadherin, also known as CD144, is a type of cadherin. It is encoded by the human gene CDH5.

Receptor-type tyrosine-protein phosphatase mu is an enzyme that in humans is encoded by the PTPRM gene.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
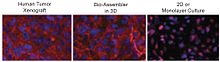
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments, a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
Magnetic 3D bioprinting is a process that utilizes biocompatible magnetic nanoparticles to print cells into 3D structures or 3D cell cultures. In this process, cells are tagged with magnetic nanoparticles, thus making them magnetic. Once magnetic, these cells can be rapidly printed into specific 3D patterns using external magnetic forces that mimic tissue structure and function.
Microfluidic cell culture integrates knowledge from biology, biochemistry, engineering, and physics to develop devices and techniques for culturing, maintaining, analyzing, and experimenting with cells at the microscale. It merges microfluidics, a set of technologies used for the manipulation of small fluid volumes within artificially fabricated microsystems, and cell culture, which involves the maintenance and growth of cells in a controlled laboratory environment. Microfluidics has been used for cell biology studies as the dimensions of the microfluidic channels are well suited for the physical scale of cells. For example, eukaryotic cells have linear dimensions between 10 and 100 μm which falls within the range of microfluidic dimensions. A key component of microfluidic cell culture is being able to mimic the cell microenvironment which includes soluble factors that regulate cell structure, function, behavior, and growth. Another important component for the devices is the ability to produce stable gradients that are present in vivo as these gradients play a significant role in understanding chemotactic, durotactic, and haptotactic effects on cells.
Cultrex Basement Membrane Extract (BME) is the trade name for a extracellular protein mixture secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells and manufactured into a hydrogel by R&D Systems, a brand of Bio-Techne. Similar to Matrigel, this hydrogel is a natural extracellular matrix that mimics the complex extracellular environment within complex tissues. It is used as a general cell culture substrate across a wide variety of research applications.
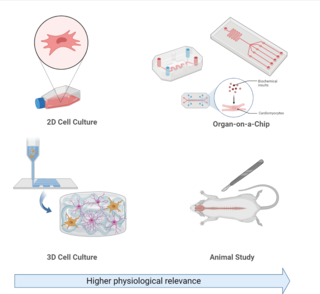
Experimental models of Alzheimer's disease are organism or cellular models used in research to investigate biological questions about Alzheimer's disease as well as develop and test novel therapeutic treatments. Alzheimer's disease is a progressive neurodegenerative disorder associated with aging, which occurs both sporadically or due to familial passed mutations in genes associated with Alzheimer's pathology. Common symptoms associated with Alzheimer's disease include: memory loss, confusion, and mood changes.