Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, oxidizing primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions, shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. However, use on an industrial scale is made difficult by its cost and its potentially explosive nature. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.

Boron trioxide or diboron trioxide is the oxide of boron with the formula B2O3. It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.

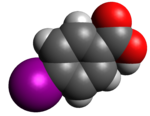
Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde. It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.

2-Pyridone is an organic compound with the formula C
5H
4NH(O). It is a colourless solid. It is well known to form hydrogen bonded dimers and it is also a classic case of a compound that exists as tautomers.

Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.

Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings. This compound has long been pursued by chemists because of its potential interest in electronic applications and was first synthesized but not cleanly isolated in 2006. Heptacene was finally fully characterized in bulk by researchers in Germany and the United States in 2017.

The long chain fatty acyl-CoA ligase is an enzyme of the ligase family that activates the oxidation of complex fatty acids. Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. The enzyme catalyzes the following reaction,

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC. Acetic acid is also known as acetyl hydroxide (AcOH).
Unlike its lighter congeners, the halogen iodine forms a number of stable organic compounds, in which iodine exhibits higher formal oxidation states than -1 or coordination number exceeding 1. These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them.
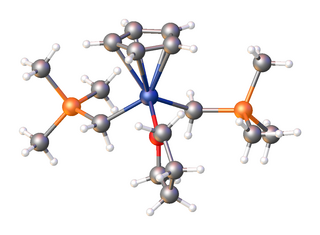
Organoscandium chemistry is an area with organometallic compounds focused on compounds with at least one carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but motivated by potential practical applications in catalysis, especially in polymerization. A common precursor is scandium chloride, especially its THF complex.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.