Methanogens are anaerobic archaea that produce methane as a byproduct of their energy metabolism, i.e., catabolism. Methane production, or methanogenesis, is the only biochemical pathway for ATP generation in methanogens. All known methanogens belong exclusively to the domain Archaea, although some bacteria, plants, and animal cells are also known to produce methane. However, the biochemical pathway for methane production in these organisms differs from that in methanogens and does not contribute to ATP formation. Methanogens belong to various phyla within the domain Archaea. Previous studies placed all known methanogens into the superphylum Euryarchaeota. However, recent phylogenomic data have led to their reclassification into several different phyla. Methanogens are common in various anoxic environments, such as marine and freshwater sediments, wetlands, the digestive tracts of animals, wastewater treatment plants, rice paddy soil, and landfills. While some methanogens are extremophiles, such as Methanopyrus kandleri, which grows between 84 and 110°C, or Methanonatronarchaeum thermophilum, which grows at a pH range of 8.2 to 10.2 and a Na+ concentration of 3 to 4.8 M, most of the isolates are mesophilic and grow around neutral pH.
Tetrahydromethanopterin is a coenzyme in methanogenesis. It is the carrier of the C1 group as it is reduced to the methyl level, before transferring to the coenzyme M.
Coenzyme B is a coenzyme required for redox reactions in methanogens. The full chemical name of coenzyme B is 7-mercaptoheptanoylthreoninephosphate. The molecule contains a thiol, which is its principal site of reaction.

5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer [6R]-5,10-methylene-THF.
In enzymology, a 3-methyl-2-oxobutanoate dehydrogenase (ferredoxin) (EC 1.2.7.7) is an enzyme that catalyzes the chemical reaction
In enzymology, an indolepyruvate ferredoxin oxidoreductase (EC 1.2.7.8) is an enzyme that catalyzes the chemical reaction

The 5,10-methenyltetrahydromethanopterin hydrogenase, the so-called iron-sulfur cluster-free hydrogenase, is an enzyme found in methanogenic archea such as Methanothermobacter marburgensis. It was discovered and first characterized by the Thauer group at the Max Planck Institute in Marburg. Hydrogenases are enzymes that either reduce protons or oxidize molecular dihydrogen.
In enzymology, a coenzyme F420 hydrogenase (EC 1.12.98.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a CoB—CoM heterodisulfide reductase (EC 1.8.98.1) is an enzyme that catalyzes the chemical reaction

In enzymology, a methylenetetrahydrofolate dehydrogenase (NADP+) (EC 1.5.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylenetetrahydromethanopterin dehydrogenase (EC 1.5.98.1) is an enzyme that catalyzes the chemical reaction
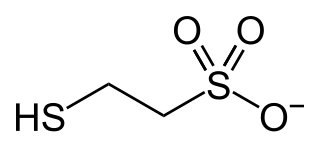
In enzymology, coenzyme-B sulfoethylthiotransferase, also known as methyl-coenzyme M reductase (MCR) or most systematically as 2-(methylthio)ethanesulfonate:N-(7-thioheptanoyl)-3-O-phosphothreonine S-(2-sulfoethyl)thiotransferase is an enzyme that catalyzes the final step in the formation of methane. It does so by combining the hydrogen donor coenzyme B and the methyl donor coenzyme M. Via this enzyme, most of the natural gas on earth was produced. Ruminants produce methane because their rumens contain methanogenic prokaryotes (Archaea) that encode and express the set of genes of this enzymatic complex.
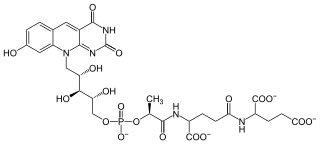
Coenzyme F420 is a family of coenzymes involved in redox reactions in a number of bacteria and archaea. It is derived from coenzyme FO (7,8-didemethyl-8-hydroxy-5-deazariboflavin) and differs by having a oligoglutamyl tail attached via a 2-phospho-L-lactate bridge. F420 is so named because it is a flavin derivative with an absorption maximum at 420 nm.

In enzymology, a methenyltetrahydromethanopterin cyclohydrolase (EC 3.5.4.27) is an enzyme that catalyzes the chemical reaction

In enzymology, a formylmethanofuran-tetrahydromethanopterin N-formyltransferase is an enzyme that catalyzes the chemical reaction

F430 is the cofactor (sometimes called the coenzyme) of the enzyme methyl coenzyme M reductase (MCR). MCR catalyzes the reaction EC 2.8.4.1 that releases methane in the final step of methanogenesis:
Malate dehydrogenase (NAD(P)+) (EC 1.1.1.299, MdH II, NAD(P)+-dependent malate dehyrogenase) is an enzyme with systematic name (S)-malate:NAD(P)+ oxidoreductase. This enzyme catalyses the following chemical reaction
(Methyl-Co methanol-specific corrinoid protein):coenzyme M methyltransferase is an enzyme with systematic name methylated methanol-specific corrinoid protein:coenzyme M methyltransferase. This enzyme catalyses the following chemical reaction
Dimethylamine-corrinoid protein Co-methyltransferase is an enzyme with systematic name dimethylamine:5-hydroxybenzimidazolylcobamide Co-methyltransferase. This enzyme catalyses the following chemical reaction
In enzymology, a formylmethanofuran dehydrogenase (EC 1.2.99.5) is an enzyme that catalyzes the chemical reaction:








