In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. However, the distinction is not clearly defined; authorities have differing views on the subject. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.
Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in several flowers and essential oils, including those of orange blossoms, jasmine, and Ziziphus mauritiana.
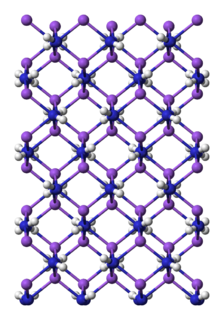
Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.
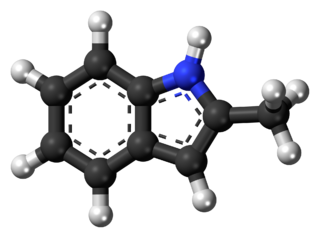
Methylketol or 2-methylindole is a mildly toxic and slightly flammable organic compound which occurs as a white solid which turns brown over time. It has chemical formula C9H9N.

7-Methylindole is a mildly toxic off-white crystalline organic compound with chemical formula C9H9N. It is used in the production of agricultural chemicals and pharmaceuticals.

1-Methylindole is an irritating, potentially toxic organic compound which occurs as a deep yellow viscous liquid with a very strong unpleasant odor. It has the chemical formula C9H9N.
A variety of isomers of methyl indole derivatives are known:

The Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. pazinaclone.

A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances, chemical compounds, or alloys. Chemical elements may or may not be included in the definition, depending on expert viewpoint.

Tabernaemontana corymbosa is a species of plant in the family Apocynaceae. It is found in Brunei, China, Indonesia, Laos, Malaysia, Myanmar, Singapore, Thailand, and Vietnam. Glossy green leaves and faintly sweet scented flower. Flowers continuously all year. Frost tolerant. Grows to about 2metres. Likes full sun to part shade. A number of cultivars are available.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.

Toxiferine is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by neostigmine

A chemical compound is a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.
The molecular formula C9H9N (molar mass: 131.17 g/mol) may refer to:

JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at CB1 and 7.0 nM at CB2. It was originally discovered by, and named after, John W. Huffman, but has subsequently been sold without his permission as an ingredient of synthetic cannabis smoking blends. Similar to the related 2'-methoxy compound JWH-250, the 2'-bromo compound JWH-249, and the 2'-methyl compound JWH-251, JWH-203 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds, and has the strongest in vitro binding affinity for the cannabinoid receptors of any compound in the phenylacetyl group.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.
The Hegedus indole synthesis is a name reaction in organic chemistry that allows for the generation of indoles through palladium(II)-mediated oxidative cyclization of ortho-alkenyl anilines. The reaction can still take place for tosyl-protected amines.














