Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in several flowers and essential oils, including those of orange blossoms, jasmine, and Ziziphus mauritiana. It has also been identified in certain cannabis varieties.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.
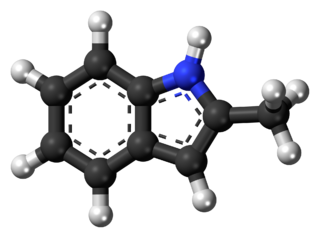
Methylketol or 2-methylindole is a mildly toxic and slightly flammable organic compound which occurs as a white solid which turns brown over time. It has chemical formula C9H9N.

7-Methylindole is a mildly toxic off-white crystalline organic compound with chemical formula C9H9N.

5-Methylindole is an irritating organic compound with chemical formula C9H9N. 5-Methylindole is used as an intermediate in the synthesis of compounds with a variety of pharmacological properties, such as staurosporine-like bisindole inhibitors of protein kinases.
A variety of isomers of methyl indole derivatives are known:

The Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.
Fog fever is a refeeding syndrome in cattle, clinically named acute bovine pulmonary emphysema and edema (ABPEE) and bovine atypical interstitial pneumonia. This veterinary disease in adult cattle follows an abrupt move from feedlot to 'foggage pasture'. Clinical signs begin within 1 to 14 days and death may follow within 2 to 4 days. The condition can affect up to 50% of the herd, and around 30% of affected cattle may die as a result. This metabolic nutritional-respiratory disturbance has also been reported in other ruminants and on a wide variety of grasses, alfalfa, rape, kale, and turnip tops.

Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. lenalidomide and pazinaclone.

Cytochrome P450 2F1 is a protein that in humans is encoded by the CYP2F1 gene.

Bopindolol (INN) is a beta blocker. It is an ester which acts as a prodrug for its active metabolite 4-(3-t-butylamino-2-hydroxypropoxy)-2-methylindole.
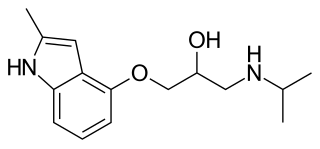
Mepindolol (Betagon) is a non-selective beta blocker. It is used to treat glaucoma.
The molecular formula C9H9N (molar mass: 131.17 g/mol) may refer to:

JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at CB1 and 7.0 nM at CB2. It was originally discovered by, and named after, John W. Huffman, but has subsequently been sold without his permission as an ingredient of synthetic cannabis smoking blends. Similar to the related 2'-methoxy compound JWH-250, the 2'-bromo compound JWH-249, and the 2'-methyl compound JWH-251, JWH-203 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds, and has the strongest in vitro binding affinity for the cannabinoid receptors of any compound in the phenylacetyl group.

The reticulated giraffe is a species/subspecies of giraffe native to the Horn of Africa. It is differentiated from other types of giraffe by its coat, which consists of large, polygonal, block-like spots, which extend onto the lower legs, tail and face. These prominent liver-red spots also show much less white between them, when compared to other giraffe species. With up to 6 meters in height, the reticulated giraffe is the largest subspecies of giraffe and the tallest land animal in general. While the reticulated giraffe may yet still be found in parts of its historic range, such as areas of Somalia and Ethiopia, its population stronghold is primarily within Kenya. There are approximately 8,500 individuals living in the wild. In both captivity and the wild, as of 2024 there are 15,785 individuals across the world.

Eudistoma is a genus of sea squirts belonging to the class Ascidiacea. It was first described in 1909 by Maurice Caullery. Originally it was thought to be a subgenus of Distoma. Eudistoma is the most species-rich genus in the family Polycitoridae, with 124 valid species as of 2014. They are found in tropical and temperate waters; some species are also found in the Antarctic and subtropical area.
Branched-chain fatty acids (BCFA) are usually saturated fatty acids with one or more methyl branches on the carbon chain. BCFAs are most often found in bacteria, but can be found in nattō, dairy, vernix caseosa of human infants and California sea lions where they may play a role in fostering the development of their intestinal microbiota. Another waxy animal material containing BCFAs is lanolin.
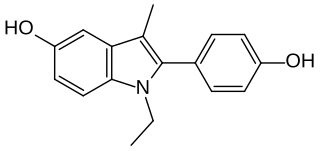
D-15414 is a nonsteroidal weak estrogen of the 2-phenylindole group which was never marketed. It is the major metabolite of the selective estrogen receptor modulator (SERM) zindoxifene (D-16726). D-15414 has high affinity for the estrogen receptor (ER) and inhibits the growth of ER-positive MCF-7 breast cancer cells in vitro. However, contradictorily, subsequent research found that the drug produced fully estrogenic effects in vitro similarly to but less actively than estradiol, with no antiestrogenic activity observed. The reason for the discrepancy between the findings is unclear, though may be due to methodology. The unexpected estrogenic activity of D-15414 may be responsible for the failure of zindoxifene in clinical trials as a treatment for breast cancer.
Olsenella scatoligenes is a Gram-positive, saccharolytic, non-spore-forming, strictly anaerobic and non-motile bacterium from the genus of Olsenella which has been isolated from faeces of a pig from the Aarhus University in Denmark.Olsenella scatoligenes produces 3-methylindole and 4-methylphenol.
The Hegedus indole synthesis is a name reaction in organic chemistry that allows for the generation of indoles through palladium(II)-mediated oxidative cyclization of ortho-alkenyl anilines. The reaction can still take place for tosyl-protected amines.















