
Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:
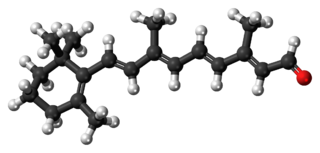
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).

Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
Ppoa or PPOA may refer to:

2,4 Dienoyl-CoA reductase also known as DECR1 is an enzyme which in humans is encoded by the DECR1 gene which resides on chromosome 8. This enzyme catalyzes the following reactions

Nitric oxide dioxygenase (EC 1.14.12.17) is an enzyme that catalyzes the conversion of nitric oxide (NO) to nitrate (NO−
3) . The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.

Peptidyl-prolyl cis-trans isomerase FKBP1A is an enzyme that in humans is encoded by the FKBP1A gene. It is also commonly referred to as FKBP-12 or FKBP12 and is a member of a family of FK506-binding proteins (FKBPs).
In enzymology, a linoleate 11-lipoxygenase (EC 1.13.11.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate diol synthase (EC 1.13.11.44) is an enzyme that catalyzes the chemical reaction
The enzyme hydroperoxide dehydratase (EC 4.2.1.92) catalyzes the chemical reaction

Microsomal triglyceride transfer protein large subunit is a protein that in humans is encoded by the MTTP gene.

cGMP-dependent protein kinase 1, alpha isozyme is an enzyme that in humans is encoded by the PRKG1 gene.
Linoleate 8R-lipoxygenase (EC 1.13.11.60, linoleic acid 8R-dioxygenase, 5,8-LDS (bifunctional enzyme), 7,8-LDS (bifunctional enzyme), 5,8-linoleate diol synthase (bifunctional enzyme), 7,8-linoleate diol synthase (bifunctional enzyme), PpoA) is an enzyme with systematic name linoleate:oxygen (8R)-oxidoreductase. This enzyme catalyses the following chemical reaction
5,8-linoleate diol synthase may refer to:
Linolenate 9R-lipoxygenase (EC 1.13.11.61, NspLOX, (9R)-LOX, linoleate 9R-dioxygenase) is an enzyme with systematic name alpha-linolenate:oxygen (9R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Linoleate 10R-lipoxygenase (EC 1.13.11.62, 10R-DOX, (10R)-dioxygenase, 10R-dioxygenase) is an enzyme with systematic name linoleate:oxygen (10R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Geraniol isomerase is an enzyme with systematic name geraniol hydroxymutase. This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
8-hydroperoxide isomerase may refer to:









