
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:
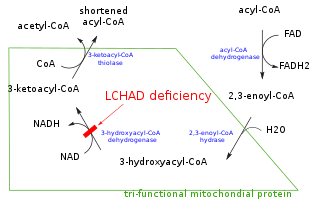
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA, which enters the citric acid cycle, and NADH and FADH2, which are co-enzymes used in the electron transport chain. It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

Phospholipase B, also known as lysophospholipase, is an enzyme with a combination of both PLA1 and PLA2 activities; that is, it can cleave acyl chains from both the sn-1 and sn-2 positions of a phospholipid. In general, it acts on lysolecithin.
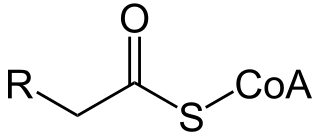
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.

The long chain fatty acyl-CoA ligase is an enzyme of the ligase family that activates the oxidation of complex fatty acids. Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. The enzyme catalyzes the following reaction,

In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.

In enzymology, a 3-oxoacyl-[acyl-carrier-protein] reductase (EC 1.1.1.100) is an enzyme that catalyzes the chemical reaction
In enzymology, an acyl-CoA oxidase (EC 1.3.3.6) is an enzyme that catalyzes the chemical reaction
In enzymology, an enoyl-[acyl-carrier-protein] reductase (NADPH, A-specific) (EC 1.3.1.39) is an enzyme that catalyzes the chemical reaction
Butyrate—CoA ligase, also known as xenobiotic/medium-chain fatty acid-ligase (XM-ligase), is an enzyme that catalyzes the chemical reaction:
The enzyme lysophospholipase (EC 3.1.1.5) catalyzes the reaction
In enzymology, a 1-acylglycerol-3-phosphate O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a 1-acylglycerophosphocholine O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a 1-alkylglycerophosphocholine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-acylglycerol-3-phosphate O-acyltransferase is an enzyme that catalyzes the chemical reaction
Sterol O-acyltransferase is an intracellular protein located in the endoplasmic reticulum that forms cholesteryl esters from cholesterol.
In enzymology, a sulfoacetaldehyde acetyltransferase is an enzyme that catalyzes the chemical reaction

2-acyl-sn-glycero-3-phosphocholines are a class of phospholipids that are intermediates in the metabolism of lipids. Because they result from the hydrolysis of an acyl group from the sn-1 position of phosphatidylcholine, they are also called 1-lysophosphatidylcholine. The synthesis of phosphatidylcholines with specific fatty acids occurs through the synthesis of 1-lysoPC. The formation of various other lipids generates 1-lysoPC as a by-product.

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.
Coenzyme A transferases (CoA-transferases) are transferase enzymes that catalyze the transfer of a coenzyme A group from an acyl-CoA donor to a carboxylic acid acceptor. Among other roles, they are responsible for transfer of CoA groups during fermentation and metabolism of ketone bodies. These enzymes are found in all three domains of life.









