Related Research Articles
Organ transplantation is a medical procedure in which an organ is removed from one body and placed in the body of a recipient, to replace a damaged or missing organ. The donor and recipient may be at the same location, or organs may be transported from a donor site to another location. Organs and/or tissues that are transplanted within the same person's body are called autografts. Transplants that are recently performed between two subjects of the same species are called allografts. Allografts can either be from a living or cadaveric source.

Liver transplantation or hepatic transplantation is the replacement of a diseased liver with the healthy liver from another person (allograft). Liver transplantation is a treatment option for end-stage liver disease and acute liver failure, although availability of donor organs is a major limitation. Liver transplantation is highly regulated, and only performed at designated transplant medical centers by highly trained transplant physicians. Favorable outcomes require careful screening for eligible recipients, as well as a well-calibrated live or deceased donor match.

Transplant rejection occurs when transplanted tissue is rejected by the recipient's immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient and by use of immunosuppressant drugs after transplant.

Everolimus, sold under the brand name Afinitor among others, is a medication used as an immunosuppressant to prevent rejection of organ transplants and as a targeted therapy in the treatment of renal cell cancer and other tumours.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Xenotransplantation, or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation, syngeneic transplantation or isotransplantation and autotransplantation. Xenotransplantation is an artificial method of creating an animal-human chimera, that is, a human with a subset of animal cells. In contrast, an individual where each cell contains genetic material from a human and an animal is called a human–animal hybrid.
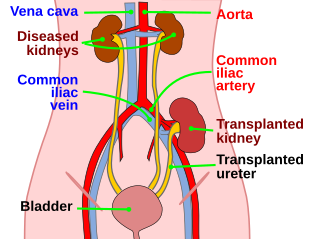
Kidney transplant or renal transplant is the organ transplant of a kidney into a patient with end-stage kidney disease (ESRD). Kidney transplant is typically classified as deceased-donor or living-donor transplantation depending on the source of the donor organ. Living-donor kidney transplants are further characterized as genetically related (living-related) or non-related (living-unrelated) transplants, depending on whether a biological relationship exists between the donor and recipient. The first successful kidney transplant was performed in 1954 by a team including Joseph Murray, the recipient's surgeon, and Hartwell Harrison, surgeon for the donor. Murray was awarded a Nobel Prize in Physiology or Medicine in 1990 for this and other work. In 2018, an estimated 95,479 kidney transplants were performed worldwide, 36% of which came from living donors.

Lung transplantation, or pulmonary transplantation, is a surgical procedure in which one or both lungs are replaced by lungs from a donor. Donor lungs can be retrieved from a living or deceased donor. A living donor can only donate one lung lobe. With some lung diseases, a recipient may only need to receive a single lung. With other lung diseases such as cystic fibrosis, it is imperative that a recipient receive two lungs. While lung transplants carry certain associated risks, they can also extend life expectancy and enhance the quality of life for those with end stage pulmonary disease.

The United Network for Organ Sharing (UNOS) is a non-profit scientific and educational organization that administers the only Organ Procurement and Transplantation Network (OPTN) in the United States, established by the U.S. Congress in 1984 by Gene A. Pierce, founder of United Network for Organ Sharing. Located in Richmond, Virginia, the organization's headquarters are situated near the intersection of Interstate 95 and Interstate 64 in the Virginia BioTechnology Research Park.
Organ procurement is a surgical procedure that removes organs or tissues for reuse, typically for organ transplantation.
Sean Patrick Pinney is an American cardiologist and the Director of both the Advanced Heart Failure and Cardiac Transplant Program and the Pulmonary Hypertension Program at Mount Sinai Medical Center in New York City.
Transplantable organs and tissues may refer to both organs and tissues that are relatively often transplanted, as well as organs and tissues which are relatively seldom transplanted. In addition to this it may also refer to possible-transplants which are still in the experimental stage.

Dale Gunnar Renlund is an American religious leader and former cardiologist who serves in the Quorum of the Twelve Apostles of the Church of Jesus Christ of Latter-day Saints. He has been a general authority of the church since 2009. Currently, he is the twelfth most senior apostle in the church.
Thymus transplantation is a form of organ transplantation where the thymus is moved from one body to another. It is used in certain immunodeficiencies, such as DiGeorge Syndrome.

A heart transplant, or a cardiac transplant, is a surgical transplant procedure performed on patients with end-stage heart failure or severe coronary artery disease when other medical or surgical treatments have failed. As of 2018, the most common procedure is to take a functioning heart, with or without both lungs, from a recently deceased organ donor and implant it into the patient. The patient's own heart is either removed and replaced with the donor heart or, much less commonly, the recipient's diseased heart is left in place to support the donor heart.

Intestine transplantation is the surgical replacement of the small intestine for chronic and acute cases of intestinal failure. While intestinal failure can oftentimes be treated with alternative therapies such as parenteral nutrition (PN), complications such as PN-associated liver disease and short bowel syndrome may make transplantation the only viable option. One of the rarest type of organ transplantation performed, intestine transplantation is becoming increasingly prevalent as a therapeutic option due to improvements in immunosuppressive regimens, surgical technique, PN, and the clinical management of pre and post-transplant patients.
Kidney paired donation (KPD), or paired exchange, is an approach to living donor kidney transplantation where patients with incompatible donors swap kidneys to receive a compatible kidney. KPD is used in situations where a potential donor is incompatible. Because better donor HLA and age matching are correlated with lower lifetime mortality and longer lasting kidney transplants, many compatible pairs are also participating in swaps to find better matched kidneys. In the United States, the National Kidney Registry organizes the majority of U.S. KPD transplants, including the largest swaps. The first large swap was a 60 participant chain in 2012 that appeared on the front page of the New York Times and the second, even larger swap, included 70 participants and was completed in 2014. Other KPD programs in the U.S. include the UNOS program, which was launched in 2010 and completed its 100th KPD transplant in 2014, and the Alliance for Paired Donation.
Edward B. Stinson is an American retired cardiothoracic surgeon living in Los Altos, United States, who assisted Norman Shumway in America's first adult human-to-human heart transplantation on 6 January 1968 at Stanford University.

Cardiac allograft vasculopathy (CAV) is a progressive type of coronary artery disease in people who have had a heart transplant. As the donor heart has lost its nerve supply there is typically no chest pain, and CAV is usually detected on routine testing. It may present with symptoms such as tiredness and breathlessness.

Ex vivo lung perfusion, EVLP, is a form of machine perfusion aimed at sustaining the active aerobic cellular metabolism of donor lungs outside the donor's body prior to lung transplantation. This medical preservation technique typically occurs within a specialised machine engineered to mimic the conditions of the natural circulatory system. The machine supplies the lung with perfusate and ventilates it using a protective mechanical ventilator under human body temperature. This allows the delivery of essential nutrients and oxygen to the donor lung, supporting metabolic functions and allowing for prolonged preservation up to 17 hours. The three major EVLP protocols at present are the Toronto protocol, Lund protocol, and Organ Care System protocol. These EVLP protocols can be effective in rendering initially rejected donor lungs suitable for transplantation through reassessment and damage repair, thus widening the donor lung pools.
References
- 1 2 3 4 West, L. J., Karamlou, T., Dipchand, A. I., Pollock-Barziv, S. M., Coles, J. G., & McCrindle, B. W. (2006). Impact on outcomes after listing and transplantation, of a strategy to accept ABO blood group-incompatible donor hearts for neonates and infants. The Journal of Thoracic and Cardiovascular Surgery, 131(2), 455–461. doi : 10.1016/j.jtcvs.2005.09.048
- 1 2 3 4 Schmoeckel, M., Däbritz, S. H., Kozlik-Feldmann, R., Wittmann, G., Christ, F., Kowalski, C., et al. (2005). Successful ABO-incompatible heart transplantation in two infants. Transplant International, 18(10), 1210–1214. doi : 10.1111/j.1432-2277.2005.00181.x
- 1 2 3 4 ABO Incompatible Heart Transplantation in Young Infants. (2009, July 30). ABO Incompatible Heart Transplantation in Young Infants. American Society of Transplantation. Retrieved from "ABO Incompatible Heart Transplantation in Young Infants | American Society of Transplantation (AST)". Archived from the original on December 20, 2013. Retrieved December 25, 2013.
- 1 2 3 West, L. J., Pollock-Barziv, S. M., Dipchand, A. I., Lee, K.-J. J., Cardella, C. J., Benson, L. N., et al. (2001). ABO-incompatible (ABOi) heart transplantation in infants. New England Journal of Medicine, 344(11), 793–800. doi : 10.1056/NEJM200103153441102
- ↑ Saczkowski, R., Dacey, C., & Bernier, P.-L. (2010). Does ABO-incompatible and ABO-compatible neonatal heart transplant have equivalent survival? Interactive cardiovascular and thoracic surgery, 10(6), 1026–1033. doi : 10.1510/icvts.2009.229757
- 1 2 3 Stewart, Z. A., Locke, J. E., Montgomery, R. A., Singer, A. L., Cameron, A. M., & Segev, D. L. (2009). ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society, 15(8), 883–893. doi : 10.1002/lt.21723
- 1 2 Almond, C. S. D., Gauvreau, K., Thiagarajan, R. R., Piercey, G. E., Blume, E. D., Smoot, L. B., et al. (2010). Impact of ABO-Incompatible Listing on Wait-List Outcomes Among Infants Listed for Heart Transplantation in the United States: A Propensity Analysis. Circulation, 121(17), 1926–1933. doi : 10.1161/CIRCULATIONAHA.109.885756
- ↑ Burch, M., & Aurora, P. (2004). Current status of paediatric heart, lung, and heart-lung transplantation. Archives of Disease in Childhood, 89(4), 386–389.
- ↑ Fan, X., Ang, A., Pollock-Barziv, S. M., Dipchand, A. I., Ruiz, P., Wilson, G., et al. (2004). Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nature medicine, 10(11), 1227–1233. doi : 10.1038/nm1126
- 1 2 3 4 Urschel, S., Larsen, I. M., Kirk, R., Flett, J., Burch, M., Shaw, N. L., et al. (2013). ABO-incompatible heart transplantation in early childhood An international multicenter study of clinical experiences and limits. The Journal of Heart and Lung Transplantation, 32(3), 285–292. doi : 10.1016/j.healun.2012.11.022
- 1 2 United Network for Organ Sharing. (2013, January 31). OPTN Policy 3.7 - Allocation of Thoracic Organs. Retrieved from "OPTN: Organ Procurement and Transplantation Network". Archived from the original on December 7, 2013. Retrieved December 25, 2013.
- ↑ Ghimire, V. (2013, March 7). Proposal to Change Pediatric Heart Allocation Policy. United Network for Organ Sharing. Retrieved from "Archived copy" (PDF). Archived from the original (PDF) on 2014-07-08. Retrieved 2013-05-05.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ McRae, Donald (2007-08-07). Every Second Counts: The Race to Transplant the First Human Heart (p. 25). Penguin Group. Kindle Edition.
- ↑ Bailey, L. L., Assaad, A. N., Trimm, R. F., Nehlsen-Cannarella, S. L., Kanakriyeh, M. S., Haas, G. S., & Jacobson, J. G. (1988). Orthotopic transplantation during early infancy as therapy for incurable congenital heart disease. Annals of Surgery, 208(3), 279.
- ↑ Klein, A. A., Lewis, C. J., & Madsen, J. C. (2011). Organ Transplantation: A Clinical Guide. p.116. Cambridge University Press.
- ↑ Everitt, M. D., Donaldson, A. E., Casper, T. C., Stehlik, J., Hawkins, J. A., Tani, L. Y., et al. (2009). Effect of ABO-incompatible listing on infant heart transplant waitlist outcomes: analysis of the United Network for Organ Sharing (UNOS) database. The Journal of Heart and Lung Transplantation, 28(12), 1254–1260. doi : 10.1016/j.healun.2009.06.024
- ↑ Roche, S. L., Burch, M., O'Sullivan, J., Wallis, J., Parry, G., Kirk, R., et al. (2007). Multicenter Experience of ABO-Incompatible Pediatric Cardiac Transplantation. American Journal of Transplantation, 0(0), 071117175452003–??? doi : 10.1111/j.1600-6143.2007.02040.x
- 1 2 Tydén, G., Hagerman, I., Grinnemo, K.-H., Svenarud, P., van der Linden, J., Kumlien, G., & Wernerson, A. (2012). Intentional ABO-incompatible heart transplantation: a case report of 2 adult patients. The Journal of Heart and Lung Transplantation, 31(12), 1307–1310. doi : 10.1016/j.healun.2012.09.011
- 1 2 Montgomery, J. R., Berger, J. C., Warren, D. S., James, N. T., Montgomery, R. A., & Segev, D. L. (2012). Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation, 93(6), 603–609. doi : 10.1097/TP.0b013e318245b2af