
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e., it has both an endocrine and a digestive exocrine function. 99% of the pancreas is exocrine and 1% is endocrine. As an endocrine gland, it functions mostly to regulate blood sugar levels, secreting the hormones insulin, glucagon, somatostatin and pancreatic polypeptide. As a part of the digestive system, it functions as an exocrine gland secreting pancreatic juice into the duodenum through the pancreatic duct. This juice contains bicarbonate, which neutralizes acid entering the duodenum from the stomach; and digestive enzymes, which break down carbohydrates, proteins and fats in food entering the duodenum from the stomach.

Beta cells (β-cells) are specialized endocrine cells located within the pancreatic islets of Langerhans responsible for the production and release of insulin and amylin. Constituting ~50–70% of cells in human islets, beta cells play a vital role in maintaining blood glucose levels. Problems with beta cells can lead to disorders such as diabetes.

The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1–2% of the pancreas volume and receive 10–15% of its blood flow. The pancreatic islets are arranged in density routes throughout the human pancreas, and are important in the metabolism of glucose.

A pancreas transplant is an organ transplant that involves implanting a healthy pancreas into a person who usually has diabetes.
Maturity-onset diabetes of the young (MODY) refers to any of several hereditary forms of diabetes mellitus caused by mutations in an autosomal dominant gene disrupting insulin production. Along with neonatal diabetes, MODY is a form of the conditions known as monogenic diabetes. While the more common types of diabetes involve more complex combinations of causes involving multiple genes and environmental factors, each forms of MODY are caused by changes to a single gene (monogenic). GCK-MODY and HNF1A-MODY are the most common forms.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
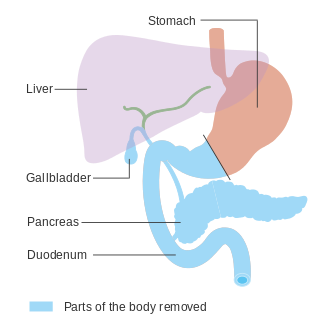
In medicine, a pancreatectomy is the surgical removal of all or part of the pancreas. Several types of pancreatectomy exist, including pancreaticoduodenectomy, distal pancreatectomy, segmental pancreatectomy, and total pancreatectomy. In total pancreatectomy, the gallbladder, distal stomach, a portion of the small intestine, associated lymph nodes and in certain cases the spleen are removed in addition to the entire pancreas. In recent years, the TP-IAT has also gained respectable traction within the medical community. These procedures are used in the management of several conditions involving the pancreas, such as benign pancreatic tumors, pancreatic cancer, and pancreatitis.

Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar for energy and it helps regulate glucose levels in the bloodstream. It results in high blood sugar levels in the body prior to treatment. The common symptoms of this elevated blood sugar are frequent urination, increased thirst, increased hunger, weight loss, and other serious complications. Additional symptoms may include blurry vision, tiredness, and slow wound healing. Symptoms typically develop over a short period of time, often a matter of weeks if not months.
Dr. A. M. James Shapiro is a British-Canadian surgeon best known for leading the clinical team that developed the Edmonton Protocol – an islet transplant procedure for the treatment of type 1 diabetes. Dr. Shapiro is Professor of Surgery, Medicine, and Surgical Oncology at the University of Alberta and the Director of the Clinical Islet Transplant Program and the Living Donor Liver Transplant Program with Alberta Health Services.
Islet transplantation is the transplantation of isolated islets from a donor pancreas into another person. It is a treatment for type 1 diabetes. Once transplanted, the islets begin to produce insulin, actively regulating the level of glucose in the blood.
Paul Eston Lacy was an anatomist and experimentalist and one of the world’s leading diabetes mellitus researchers. He is often credited as the originator of islet transplantation.
R. Paul Robertson is an American endocrinologist and former president and scientific director of the Pacific Northwest Diabetes Research Institute (PNDRI). He is a professor of medicine at the University of Minnesota. Robertson was the 2009 president, Medicine & Science of the Volunteer Board for the American Diabetes Association. His research interests focus on glucose regulation of pancreatic islet gene expression and the abnormal consequences on gene expression caused by glucose toxicity. He is also involved in metabolic studies of type 1 diabetic patients who have successfully received pancreas and pancreatic islet transplantation. He was elected to be the editor-in-chief for the Journal of Clinical Endocrinology and Metabolism (JCEM) of the Endocrine Society beginning January 2015.

The insulin concentration in blood increases after meals and gradually returns to basal levels during the next 1–2 hours. However, the basal insulin level is not stable. It oscillates with a regular period of 3-6 min. After a meal the amplitude of these oscillations increases but the periodicity remains constant. The oscillations are believed to be important for insulin sensitivity by preventing downregulation of insulin receptors in target cells. Such downregulation underlies insulin resistance, which is common in type 2 diabetes. It would therefore be advantageous to administer insulin to diabetic patients in a manner mimicking the natural oscillations. The insulin oscillations are generated by pulsatile release of the hormone from the pancreas. Insulin originates from beta cells located in the islets of Langerhans. Since each islet contains up to 2000 beta cells and there are one million islets in the pancreas it is apparent that pulsatile secretion requires sophisticated synchronization both within and among the islets of Langerhans.
Insulitis is an inflammation of the islets of Langerhans, a collection of endocrine tissue located in the pancreas that helps regulate glucose levels, and is classified by specific targeting of immune cell infiltration in the islets of Langerhans. This immune cell infiltration can result in the destruction of insulin-producing beta cells of the islets, which plays a major role in the pathogenesis, the disease development, of type 1 and type 2 diabetes. Insulitis is present in 19% of individuals with type 1 diabetes and 28% of individuals with type 2 diabetes. It is known that genetic and environmental factors contribute to insulitis initiation, however, the exact process that causes it is unknown. Insulitis is often studied using the non-obese diabetic (NOD) mouse model of type 1 diabetes. The chemokine family of proteins may play a key role in promoting leukocytic infiltration into the pancreas prior to pancreatic beta-cell destruction.
Transplantable organs and tissues may refer to both organs and tissues that are relatively often transplanted, as well as organs and tissues which are relatively seldom transplanted. In addition to this it may also refer to possible-transplants which are still in the experimental stage.

The condition known today as diabetes is thought to have been described in the Ebers Papyrus. Ayurvedic physicians first noted the sweet taste of diabetic urine, and called the condition madhumeha. The term diabetes traces back to Demetrius of Apamea. For a long time, the condition was described and treated in traditional Chinese medicine asxiāo kě. Physicians of the medieval Islamic world, including Avicenna, have also written on diabetes. Early accounts often referred to diabetes as a disease of the kidneys. In 1674, Thomas Willis suggested that diabetes may be a disease of the blood. Johann Peter Frank is credited with distinguishing diabetes mellitus and diabetes insipidus in 1794.
Bernhard J. Hering is professor of surgery and medicine and executive director of the Schulze Diabetes Institute at the University of Minnesota, where he serves as Vice Chair of Translational Medicine in the Medical School's Department of Surgery and where he holds the McKnight Presidential Chair in Transplantation Science and the Jeffrey Dobbs and David Sutherland, MD, PhD Chair in Diabetes Research.
Rainer W.G. Gruessner is a German-born American general surgeon and transplant surgeon, most noted as a surgical pioneer for his clinical and research innovations. Gruessner was the first transplant surgeon to perform all types of abdominal transplants from living donors.
Brockmann body is an endocrine organ in some teleost fish, and is composed of a collection of islet tissues. The islet tissues are in turn composed of endocrine cells which are the principal sites of insulin synthesis. They are distributed around the spleen and the large intestine. They also secrete other hormones such as glucagon and somatostatin. Hence, Brochmann body is the centre of control of blood glucose level in these fishes. Glucagon is also produced from the intestine, but Brockmann body is the major source. Increased level of glucose stimulate the Brockmann body to release insulin, while inhibiting glucagon. Somatostatin released from Brockmann body inhibits cells to produce insulin and glucagon. In addition it inhibits release of growth hormone from the pituitary. It is named after a German physician Heinrich Brochmann who discovered it in 1848.
Diabetes mellitus (DM) is a type of metabolic disease characterized by hyperglycemia. It is caused by either defected insulin secretion or damaged biological function, or both. The high-level blood glucose for a long time will lead to dysfunction of a variety of tissues.












