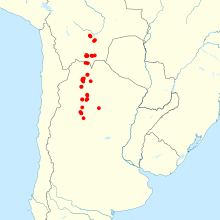
Philip Myers's akodont is a recently described species of grass mouse from Misiones Province, Argentina. Like other grass mice, A. philipmyersi is a small, non-descript, greyish-brown mouse with prominent ears. The species was recognized as distinct from other grass mice on the basis of unique features of karyology, genetic sequence, cranial measurements, and general morphology.

Akodon is a genus consisting of South American grass mice. They mostly occur south of the Amazon Basin and along the Andes north to Venezuela, but are absent from much of the basin itself, the far south of the continent, and the lowlands west of the Andes. Akodon is one of the most species-rich genera of Neotropical rodents. Species of Akodon are known to inhabit a variety of habitats from tropical and tropical moist forests to altiplano and desert. Fossils are known from the late Pliocene onwards.

Necromys is a genus of South American sigmodontine rodents allied to Akodon. This genus has also been known as Cabreramys or more recently Bolomys, and the northern grass mouse has recently been transferred from Akodon.

The pygmy fruit bat, also known as the grey fruit bat, is a species of megabat.
Oligoryzomys chacoensis, also known as the Chacoan colilargo or Chacoan pygmy rice rat, is a rodent species from South America. It is found in the Gran Chaco region of southeastern Bolivia, southwestern Brazil, Paraguay, and northeastern Argentina. Its karyotype has 2n = 58 and FNa = 74.

Oligoryzomys flavescens, also known as the flavescent colilargo or yellow pygmy rice rat is a species of rodent in the genus Oligoryzomys of family Cricetidae. It is found in southern South America, occurring in southern Brazil, Paraguay, Uruguay, and northeastern Argentina. Its karyotype has 2n = 64-66 and FNa = 66–70.

Lundomys molitor, also known as Lund's amphibious rat or the greater marsh rat, is a semiaquatic rat species from southeastern South America.

Pseudoryzomys simplex, also known as the Brazilian false rice rat or false oryzomys, is a species of rodent in the family Cricetidae from south-central South America. It is found in lowland palm savanna and thorn scrub habitats. It is a medium-sized species, weighing about 50 grams (1.8 oz), with gray–brown fur, long and narrow hindfeet, and a tail that is about as long as the head and body. The IUCN has assessed its conservation status as being of least concern, although almost nothing is known about its diet or reproduction.

Akodon albiventer, also known as the white-bellied grass mouse or white-bellied akodont, is a species of rodent in the family Cricetidae. It is found in the Andean highlands from southeastern Peru to southwestern Bolivia, northwestern Argentina, and far northeastern Chile at elevations from 2400 m to over 5000 m.
Akodon boliviensis, also known as the Bolivian grass mouse or Bolivian akodont, is a species of rodent in the family Cricetidae. It is found in the Andes from southeastern Peru through Bolivia into northwestern Argentina.
Akodon budini, also known as Budin's akodont or Budin's grass mouse, is a species of rodent in the family Cricetidae. It is found in the Andes of northwestern Argentina and adjacent Bolivia. The species is named after Emilio Budin, an Argentine specimen collector who worked with Oldfield Thomas.

Akodon spegazzinii, also known as Spegazzini's akodont or Spegazzini's grass mouse, is a rodent in the genus Akodon found in northwestern Argentina. It occurs in grassland and forest at 400 to 3,500 m above sea level. After the species was first named in 1897, several other names were given to various populations now included in A. spegazzinii. They are now all recognized as part of a single, widespread and variable species. Akodon spegazzinii is related to Akodon boliviensis and other members of the A. boliviensis species group. It reproduces year-round. Because it is widely distributed and common, Akodon spegazzinii is listed as "least concern" on the IUCN Red List.

Akodon sylvanus, also known as the forest grass mouse or woodland akodont, is a species of rodent in the family Cricetidae. It is found only in a small part of northwestern Argentina.
Oligoryzomys destructor, also known as Tschudi's colilargo or the destructive pygmy rice rat, is a species of rodent in the genus Oligoryzomys of family Cricetidae. It is found along the eastern Andes from southern Colombia, through Ecuador, Peru, and Bolivia into northern Argentina. Its karyotype has 2n = 60 and FNa = 76.
The southern climbing mouse is a species of rodent in the family Cricetidae. It is found in Argentina and Bolivia in forested valleys and on slopes on the eastern side of the Andes Mountains.

The plains viscacha rat, plains vizcacha rat, red viscacha rat, or red vizcacha rat is a species of rodent in the family Octodontidae native to Argentina. It is one of three species in the genus Tympanoctomys.
Reigomys primigenus is an extinct oryzomyine rodent known from Pleistocene deposits in Tarija Department, southeastern Bolivia. It is known from a number of isolated jaws and molars which show that its molars were almost identical to those of the living Lundomys. On the other hand, the animal possesses a number of derived traits of the palate which document a closer relationship to living Holochilus, the genus of South American marsh rats, and for this reason it was placed in the genus Holochilus when it was first described in 1996. The subsequent discoveries of Noronhomys and Carletonomys, which may be more closely related to extant Holochilus than H. primigenus is, have cast its placement in Holochilus into doubt, and it was ultimately made the type species of a separate genus, Reigomys.

Abrotrichini, also known as the Andean clade or southern Andean clade, is a tribe of rodents in the subfamily Sigmodontinae. It includes about fifteen species in four genera, distributed in South America from southern Peru to southernmost South America, including the Patagonian steppes. The earliest known fossils are from the Pliocene of Argentina.

Cryptonanus is a genus of opossums from South America. It includes five species found from Bolivia to Uruguay and eastern Brazil, one of which is now extinct. Although the first species were discovered in 1931, the genus was not recognized as distinct from Gracilinanus until 2005. It includes small opossums with generally grayish, sometimes reddish, fur that are mainly distinguished from other opossums by characters of the skull.
Akodon polopi is a species of rodent in the family Cricetidae. It is found in Argentina.