Interleukins (ILs) are a group of cytokines that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related proteins.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.
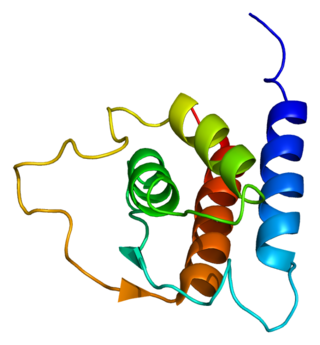
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.
Interleukin 33 (IL-33) is a protein that in humans is encoded by the IL33 gene.

Interleukin-25 (IL-25) – also known as interleukin-17E (IL-17E) – is a protein that in humans is encoded by the IL25 gene on chromosome 14. IL-25 was discovered in 2001 and is made up of 177 amino acids.

Interleukin-22 (IL-22) is protein that in humans is encoded by the IL22 gene.
Sir John Stewart Savill, FRS, FMedSci is the Chief Executive of the Medical Research Council (MRC) in the UK and the Head of the College of Medicine and Veterinary Medicine and a Vice Principal of the University of Edinburgh.
Chronic systemic inflammation (SI) is the result of release of pro-inflammatory cytokines from immune-related cells and the chronic activation of the innate immune system. It can contribute to the development or progression of certain conditions such as cardiovascular disease, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease, autoimmune and neurodegenerative disorders, and coronary heart disease.

John J. O'Shea is an American physician and immunologist.
Interleukin 1 receptor-like 1, also known as IL1RL1 and ST2, is a protein that in humans is encoded by the IL1RL1 gene.
The nuocyte is a cell of the innate immune system that plays an important role in type 2 immune responses that are induced in response to helminth worm infection or in conditions such as asthma and atopic disease. Nuocytes are amongst the first cells activated in type 2 immune responses and are thought to play important roles in activating and recruiting other cells types through their production of type 2 cytokines interleukin 4, 5 and 13. Nuocytes have been observed to proliferate in the presence of interleukin 7 (IL-7) in vitro. Nuocytes contribute to the expulsion of helminth worms and to the pathology of colitis and allergic airways disease.

Interleukin 23 (IL-23) is a heterodimeric cytokine composed of an IL-12B (IL-12p40) subunit and an IL-23A (IL-23p19) subunit. IL-23 is part of the IL-12 family of cytokines. The functional receptor for IL-23 consists of a heterodimer between IL-12Rβ1 and IL-23R.
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.
Brigitta Stockinger, FMedSci, FRS, is a molecular immunologist in the Francis Crick Institute in London. Stockinger's lab focus on understanding how certain immune cells, called T cells, develop and function as well as investigating how diet and other environmental factors can affect the way the immune system works.

Luke Anthony John O'Neill is an Irish biochemist. He has been a professor of biochemistry in the School of Biochemistry and Immunology at Trinity College Dublin since 2009.

ILC2 cells, or type 2 innate lymphoid cells are a type of innate lymphoid cell. Not to be confused with the ILC. They are derived from common lymphoid progenitor and belong to the lymphoid lineage. These cells lack antigen specific B or T cell receptor because of the lack of recombination activating gene. ILC2s produce type 2 cytokines and are involved in responses to helminths, allergens, some viruses, such as influenza virus and cancer.

Type 3 innate lymphoid cells (ILC3) are immune cells from the lymphoid lineage that are part of the innate immune system. These cells participate in innate mechanisms on mucous membranes, contributing to tissue homeostasis, host-commensal mutualism and pathogen clearance. They are part of a heterogeneous group of innate lymphoid cells, which is traditionally divided into three subsets based on their expression of master transcription factors as well as secreted effector cytokines - ILC1, ILC2 and ILC3.
Charles Bangham holds the Chair in Immunology at Imperial College London.
Caetano Maria Pacheco Pais dos Reis e Sousa is a senior group leader at the Francis Crick Institute and a professor of Immunology at Imperial College London.
Judith Elizabeth Allen is a British scientist who is Professor of Immunobiology at the University of Manchester. She is an expert on macrophages activated during helminthiasis and was elected Fellow of the Royal Society (FRS) in 2023. She has also done extensive work into type 2 immunity and was awarded Honorary Professor at the University of Edinburgh in 2016.










