In science and engineering the study of high pressure examines its effects on materials and the design and construction of devices, such as a diamond anvil cell, which can create high pressure. High pressure usually means pressures of thousands (kilobars) or millions (megabars) of times atmospheric pressure.
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.
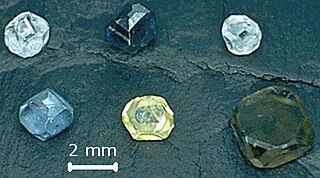
Lab-grown diamond is diamond that is produced in a controlled technological process. Unlike diamond simulants, synthetic diamonds are composed of the same material as naturally formed diamonds—pure carbon crystallized in an isotropic 3D form—and share identical chemical and physical properties.

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
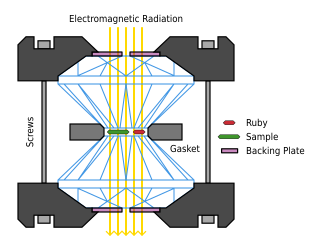
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It enables the compression of a small (sub-millimeter-sized) piece of material to extreme pressures, typically up to around 100–200 gigapascals, although it is possible to achieve pressures up to 770 gigapascals.

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal that is transparent to opaque and which is generally isotropic. Diamond is the hardest naturally occurring material known. Yet, due to important structural brittleness, bulk diamond's toughness is only fair to good. The precise tensile strength of bulk diamond is little known; however, compressive strength up to 60 GPa has been observed, and it could be as high as 90–100 GPa in the form of micro/nanometer-sized wires or needles, with a corresponding maximum tensile elastic strain in excess of 9%. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.
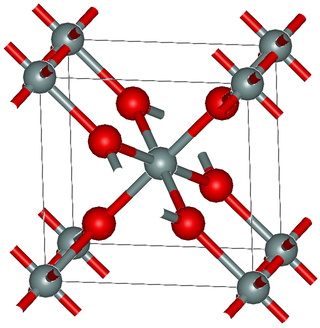
Stishovite is an extremely hard, dense tetragonal form (polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.
Post-perovskite (pPv) is a high-pressure phase of magnesium silicate (MgSiO3). It is composed of the prime oxide constituents of the Earth's rocky mantle (MgO and SiO2), and its pressure and temperature for stability imply that it is likely to occur in portions of the lowermost few hundred km of Earth's mantle.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.
Pressure experiments are experiments performed at pressures lower or higher than atmospheric pressure, called low-pressure experiments and high-pressure experiments, respectively. Pressure experiment are necessary because substances behave differently at different pressures. For example, water boils at a lower temperature at lower pressures. The equipment used for pressure experiments depends on whether the pressure is to be increased or decreased and by how much. A vacuum pump is used to remove the air out of a vacuum vessel for low-pressure experiments. High-pressures can be created with a piston-cylinder apparatus, up to 5 GPa and ~2000 °C. The piston is shifted with hydraulics, decreasing the volume inside the confining cylinder and increasing the pressure. For higher pressures, up to 25 GPa, a multi-anvil cell is used and for even higher pressures the diamond anvil cell. The diamond anvil cell is used to create extremely high pressures, as much as a million atmospheres, though only over a small area. The current record is 560 GPa, but the sample size is confined to the order of tens of micrometres.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).

Ho-Kwang (Dave) Mao is a Chinese-American geologist. He is the director of the Center for High Pressure Science and Technology Advanced Research in Shanghai, China. He was a staff scientist at Geophysical Laboratory of the Carnegie Institution for Science for more than 30 years. Mao is a recognized leading scientist in high pressure geosciences and physical science. There are two minerals named after him, Davemaoite and Maohokite.
Solid hydrogen is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen's melting point of 14.01 K. It was collected for the first time by James Dewar in 1899 and published with the title "Sur la solidification de l'hydrogène" in the Annales de Chimie et de Physique, 7th series, vol. 18, Oct. 1899. Solid hydrogen has a density of 0.086 g/cm3 making it one of the lowest-density solids.
Mineral physics is the science of materials that compose the interior of planets, particularly the Earth. It overlaps with petrophysics, which focuses on whole-rock properties. It provides information that allows interpretation of surface measurements of seismic waves, gravity anomalies, geomagnetic fields and electromagnetic fields in terms of properties in the deep interior of the Earth. This information can be used to provide insights into plate tectonics, mantle convection, the geodynamo and related phenomena.

Iron–hydrogen alloy, also known as iron hydride, is an alloy of iron and hydrogen and other elements. Because of its lability when removed from a hydrogen atmosphere, it has no uses as a structural material.
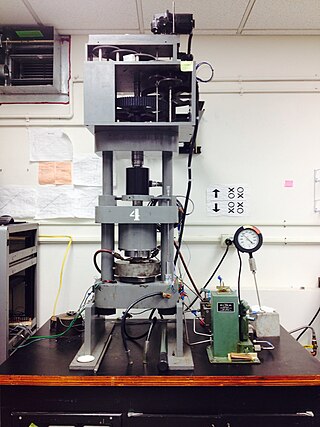
Griggs apparatus, also referred to as a Griggs rig, is a modified piston cylinder high pressure apparatus used to create an environment of high pressure, high temperature and to impart a deviatoric stress on a sample of material. It was conceived in the 1960s.
The D-DIA or deformation-DIA is an apparatus used for high pressure and high temperature deformation experiments. The advantage of this apparatus is the ability to apply pressures up to approximately 15 GPa while independently creating uniaxial strains up to 50%.

The piston-cylinder apparatus is a solid media device, used in Geosciences and Material Sciences, for generating simultaneously high pressure and temperature. Modifications of the normal set-up can push these limits to even higher pressures and temperatures. A particular type of piston-cylinder, called Griggs apparatus, is also able to add a deviatoric stress on the sample.
The geochemistry of carbon is the study of the transformations involving the element carbon within the systems of the Earth. To a large extent this study is organic geochemistry, but it also includes the very important carbon dioxide. Carbon is transformed by life, and moves between the major phases of the Earth, including the water bodies, atmosphere, and the rocky parts. Carbon is important in the formation of organic mineral deposits, such as coal, petroleum or natural gas. Most carbon is cycled through the atmosphere into living organisms and then respirated back into the atmosphere. However an important part of the carbon cycle involves the trapping of living matter into sediments. The carbon then becomes part of a sedimentary rock when lithification happens. Human technology or natural processes such as weathering, or underground life or water can return the carbon from sedimentary rocks to the atmosphere. From that point it can be transformed in the rock cycle into metamorphic rocks, or melted into igneous rocks. Carbon can return to the surface of the Earth by volcanoes or via uplift in tectonic processes. Carbon is returned to the atmosphere via volcanic gases. Carbon undergoes transformation in the mantle under pressure to diamond and other minerals, and also exists in the Earth's outer core in solution with iron, and may also be present in the inner core.
Alvin Van Valkenburg, Jr. was an experimental physicist, geologist, geochemist, and inventor, known as one of the four co-inventors of the diamond anvil cell (DAC).











