Related Research Articles
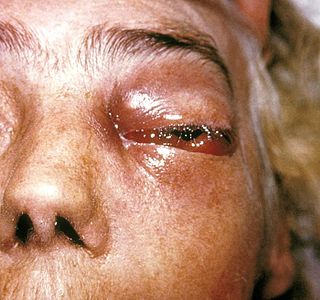
Zygomycosis is the broadest term to refer to infections caused by bread mold fungi of the zygomycota phylum. However, because zygomycota has been identified as polyphyletic, and is not included in modern fungal classification systems, the diseases that zygomycosis can refer to are better called by their specific names: mucormycosis, phycomycosis and basidiobolomycosis. These rare yet serious and potentially life-threatening fungal infections usually affect the face or oropharyngeal cavity. Zygomycosis type infections are most often caused by common fungi found in soil and decaying vegetation. While most individuals are exposed to the fungi on a regular basis, those with immune disorders (immunocompromised) are more prone to fungal infection. These types of infections are also common after natural disasters, such as tornadoes or earthquakes, where people have open wounds that have become filled with soil or vegetative matter.

Sporothrix schenckii, a fungus that can be found worldwide in the environment, is named for medical student Benjamin Schenck, who in 1896 was the first to isolate it from a human specimen. The species is present in soil as well as in and on living and decomposing plant material such as peat moss. It can infect humans as well as animals and is the causative agent of sporotrichosis, commonly known as "rose handler's disease." The most common route of infection is the introduction of spores to the body through a cut or puncture wound in the skin. Infection commonly occurs in otherwise healthy individuals but is rarely life-threatening and can be treated with antifungals. In the environment it is found growing as filamentous hyphae. In host tissue it is found as a yeast. The transition between the hyphal and yeast forms is temperature dependent making S. schenckii a thermally dimorphic fungus.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Cochliobolus lunatus is a fungal plant pathogen that can cause disease in humans and other animals. The anamorph of this fungus is known as Curvularia lunata, while C. lunatus denotes the teleomorph or sexual stage. They are, however, the same biological entity. C. lunatus is the most commonly reported species in clinical cases of reported Cochliobolus infection.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.
Pathogenic fungi are fungi that cause disease in humans or other organisms. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. Approximately 300 fungi are known to be pathogenic to humans; their study is called "medical mycology". Fungal infections kill more people than either tuberculosis or malaria—about 2 million people per year.

Lomentospora prolificans is an emerging opportunistic fungal pathogen that causes a wide variety of infections in immunologically normal and immunosuppressed people and animals. It is resistant to most antifungal drugs and infections are often fatal. Drugs targeting the Class II dihydroorotate dehydrogenase (DHODH) proteins of L. prolificans, Scedosporium, Aspergillus and other deadly moulds are the basis for at least one new therapy, Olorofim, which is currently in phase 2b clinical trials and has received breakthrough status by FDA. For information on all DHODH proteins, please see Dihydroorotate dehydrogenase.
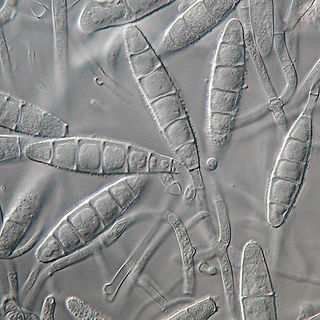
Microsporum gypseum is a soil-associated dermatophyte that occasionally is known to colonise and infect the upper dead layers of the skin of mammals. The name refers to an asexual "form-taxon" that has been associated with four related biological species of fungi: the pathogenic taxa Arthroderma incurvatum, A. gypsea, A. fulva and the non-pathogenic saprotroph A. corniculata. More recent studies have restricted M. gypseum to two teleomorphic species A. gypseum and A. incurvatum. The conidial states of A. fulva and A. corniculata have been assigned to M. fulvum and M. boullardii. Because the anamorphic states of these fungi are so similar, they can be identified reliably only by mating. Two mating strains have been discovered, "+" and "–". The classification of this species has been based on the characteristically rough-walled, blunt, club-shaped, multicelled macroconidia. Synonyms include Achorion gypseum, Microsporum flavescens, M. scorteum, and M. xanthodes. There has been past nomenclatural confusion in the usage of the generic names Microsporum and Microsporon.

Exophiala dermatitidis is a thermophilic black yeast, and a member of the Herpotrichiellaceae. While the species is only found at low abundance in nature, metabolically active strains are commonly isolated in saunas, steam baths, and dish washers. Exophiala dermatitidis only rarely causes infection in humans, however cases have been reported around the world. In East Asia, the species has caused lethal brain infections in young and otherwise healthy individuals. The fungus has been known to cause cutaneous and subcutaneous phaeohyphomycosis, and as a lung colonist in people with cystic fibrosis in Europe. In 2002, an outbreak of systemic E. dermatitidis infection occurred in women who had received contaminated steroid injections at North Carolina hospitals.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.
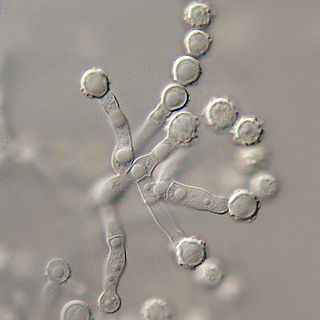
Microascus brevicaulis is a microfungus in the Ascomycota. It is the teleomorph form of Scopulariopsis brevicaulis.Microascus brevicaulis occurs world-wide as a saprotroph in soil, a common agent of biodeterioration, an irregular plant pathogen, and an occasional agent of human nail infection.
Scedosporiosis is the general name for any mycosis - i.e., fungal infection - caused by a fungus from the genus Scedosporium. Current population-based studies suggest Scedosporium prolificans and Scedosporium apiospermum to be among the most common infecting agents from the genus, although infections caused by other members thereof are not unheard of. The latter is an asexual form (anamorph) of another fungus, Pseudallescheria boydii. The former is a “black yeast”, currently not characterized as well, although both of them have been described as saprophytes.

Neoscytalidium dimidiatum was first described in 1933 as Hendersonula toruloidea from diseased orchard trees in Egypt. Decades later, it was determined to be a causative agent of human dermatomycosis-like infections and foot infections predominantly in tropical areas; however the fungus is considered to be widespread. A newer name, Scytalidium dimidiatum, was applied to a synanamorph of Nattrassia mangiferae, otherwise known as Neofusicoccum mangiferae. Substantial confusion has arisen in the literature on this fungus resulting from the use of multiple different names including Torula dimidiata, Fusicoccum dimidiatum, Scytalidium dimidiatum, and Hendersonula toruloidea. Additionally, Scytalidium lignicola and Scytalidium lignicolum are often considered earlier names of N. dimidiatum.
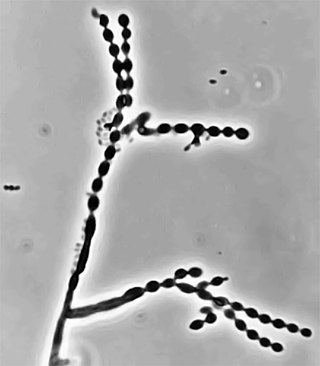
Cladophialophora carrionii is a melanized fungus in the genus Cladophialophora that is associated with decaying plant material like cacti and wood. It is one of the most frequent species of Cladophialophora implicated in human disease. Cladophialophora carrionii is a causative agent of chromoblastomycosis, a subcutaneous infection that occurs in sub-tropical areas such as Madagascar, Australia and northwestern Venezuela. Transmission occurs through traumatic implantation of plant material colonized by C. carrionii, mainly infecting rural workers. When C. carrionii infects its host, it transforms from a mycelial state to a muriform state to better tolerate the extreme conditions in the host's body.
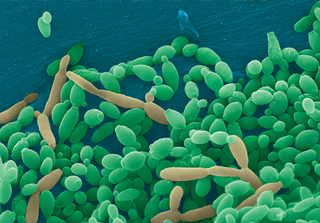
Candida tropicalis is a species of yeast in the genus Candida. It is a common pathogen in neutropenic hosts, in whom it may spread through the bloodstream to peripheral organs. For invasive disease, treatments include amphotericin B, echinocandins, or extended-spectrum triazole antifungals.

Epidermophyton floccosum is a filamentous fungus that causes skin and nail infections in humans. This anthropophilic dermatophyte can lead to diseases such as tinea pedis, tinea cruris, tinea corporis and onychomycosis. Diagnostic approaches of the fungal infection include physical examination, culture testing, and molecular detection. Topical antifungal treatment, such as the use of terbinafine, itraconazole, voriconazole, and ketoconazole, is often effective.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
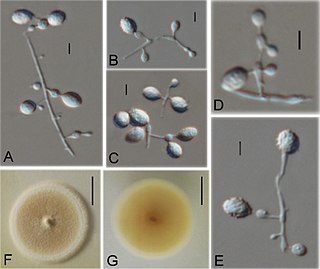
Ctenomyces serratus is a keratinophilic fungal soil saprotroph classified by the German mycologist, Michael Emil Eduard Eidam in 1880, who found it growing on an old decayed feather. Many accounts have shown that it has a global distribution, having been isolated in select soils as well as on feathers and other substrates with high keratin content. It has also been found in indoor dust of hospitals and houses in Kanpur, Northern India and as a common keratinophilic soil fungus in urban Berlin. This species has been associated with nail infections in humans as well as skin lesions and slower hair growth in guinea pigs.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
References
- 1 2 3 Sugiura, Yoshitsugu (1 March 2010). "Arthrographis kalrae, a rare causal agent of onychomycosis, and its occurrence in natural and commercially available soils". Medical Mycology. 48 (2): 384–389. doi:10.3109/13693780903219014. PMID 20141374 . Retrieved 23 November 2018.
- 1 2 CJ, Campoverde Espinoza (2017). "pulmonary infection by Arthrographis kalrae in patient with chronic granulomatous disease". Arch Argent Pediatr. 115 (6): 458–61. doi: 10.5546/aap.2017.e458 . PMID 29087135.
- 1 2 3 4 5 6 7 Sigler, L; Carmichael, JW (1976). "Taxonomy of Malbranchea and some other Hyphomycetes with arthroconidia". Mycotaxon. 4 (2): 349–488.
- 1 2 Barron, George L. (1968). The genera of Hyphomycetes from soil. Baltimore, MD: Williams & Wilkins. ISBN 9780882750040.
- 1 2 3 4 A, Giraldo (2014). "Phylogenetic circumscription of Arthrographis (Eremocetaceae, Dithideomycetes)". Persoonia. 32: 102–114. doi:10.3767/003158514X680207. PMC 4150071 . PMID 25264385.
- 1 2 3 4 5 6 Howard, Dexter H. (2007). Pathogenic fungi in humans and animals (2nd ed.). New York, NY: Dekker. ISBN 978-0824706838.
- 1 2 J, Denis (2016). "First case of Arthrographis kalrae fungemia in a patient with cystic fibrosis". Med Mycol Case Rep. 14: 8–11. doi:10.1016/j.mmcr.2016.11.002. PMC 5154970 . PMID 27995052.
- ↑ G, Plaza (1998). "Effect of cadmium on growth of potentially pathogenic soil fungi". Mycopathologia. 141 (2): 93–100. doi:10.1023/A:1006991306756. PMID 9786763. S2CID 9564573.
- 1 2 3 4 5 Rippon, John Willard (1988). Medical mycology: the pathogenic fungi and the pathogenic actinomycetes (3rd ed.). Philadelphia, PA: Saunders. ISBN 978-0721624440.
- ↑ M, Sandoval-Denis (2014). "In vitro antifungal susceptibility of clinical isolates of Arthrographis kalrae, a poorly known opportunistic fungus". Mycoses. 57 (4): 247–8. doi:10.1111/myc.12151. PMID 24147779. S2CID 2758414.
- 1 2 3 LA, nagashima (2016). "Immunomodulation over the course of experimental Arthrographis kalrae infection in mice". Comp Immunol Microbiol Infect Dis. 48: 79–86. doi:10.1016/j.cimid.2016.08.003. PMID 27638123.
- ↑ PV, Chin-Hong (2001). "Invasive fungal sinusitis and meningitis due to Arthrographis kalrae in a patient with AIDS". J. Clin. Microbiol. 39 (2): 804–7. doi:10.1128/JCM.39.2.804-807.2001. PMC 87827 . PMID 11158158.
- ↑ LA, nagashima (2014). "Arthrographis Kalrae soluble antigens present hemolytic and cytotoxic actitvities". Comp Immunol Microbiol Infect Dis. 37 (5–6): 306–11. doi:10.1016/j.cimid.2014.09.002. PMID 25449999.
- 1 2 DE, Corzo-Leon (2015). "Epidemiology and outcomes of invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients in the era of anti fungal prophylaxis: a single-centre study with focus on emerging pathogens". Mycoses. 58 (6): 325–36. doi:10.1111/myc.12318. PMID 25808822. S2CID 206200157.