
Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal or glial cell after it has performed its function of transmitting a neural impulse.
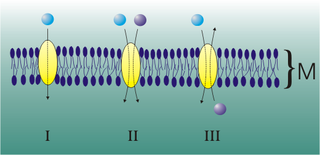
Mediated transport refers to transport mediated by a membrane transport protein. Substances in the human body may be hydrophobic, electrophilic, contain a positively or negatively charge, or have another property. As such there are times when those substances may not be able to pass over the cell membrane using protein-independent movement. The cell membrane is imbedded with many membrane transport proteins that allow such molecules to travel in and out of the cell. There are three types of mediated transporters: uniport, symport, and antiport. Things that can be transported are nutrients, ions, glucose, etc, all depending on the needs of the cell. One example of a uniport mediated transport protein is GLUT1. GLUT1 is a transmembrane protein, which means it spans the entire width of the cell membrane, connecting the extracellular and intracellular region. It is a uniport system because it specifically transports glucose in only one direction, down its concentration gradient across the cell membrane.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion, active transport, osmosis, or reverse diffusion. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. Examples of channel/carrier proteins include the GLUT 1 uniporter, sodium channels, and potassium channels. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well.
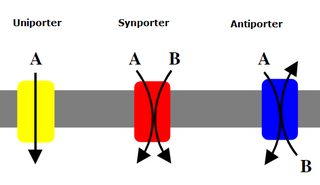
Uniporters, also known as solute carriers or facilitated transporters, are a type of membrane transport protein that passively transports solutes across a cell membrane. It uses facilitated diffusion for the movement of solutes down their concentration gradient from an area of high concentration to an area of low concentration. Unlike active transport, it does not require energy in the form of ATP to function. Uniporters are specialized to carry one specific ion or molecule and can be categorized as either channels or carriers. Facilitated diffusion may occur through three mechanisms: uniport, symport, or antiport. The difference between each mechanism depends on the direction of transport, in which uniport is the only transport not coupled to the transport of another solute.

An antiporter is an integral membrane protein that uses secondary active transport to move two or more molecules in opposite directions across a phospholipid membrane. It is a type of cotransporter, which means that uses the energetically favorable movement of one molecule down its electrochemical gradient to power the energetically unfavorable movement of another molecule up its electrochemical gradient. This is in contrast to symporters, which are another type of cotransporter that moves two or more ions in the same direction, and primary active transport, which is directly powered by ATP.

Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable coupled or cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.
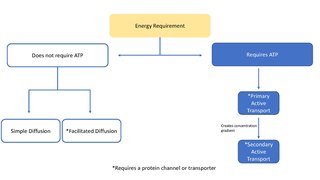
In biology, an ion transporter is a transmembrane protein that moves ions across a biological membrane to accomplish many different biological functions, including cellular communication, maintaining homeostasis, energy production, etc. There are different types of transporters including pumps, uniporters, antiporters, and symporters. Active transporters or ion pumps are transporters that convert energy from various sources—including adenosine triphosphate (ATP), sunlight, and other redox reactions—to potential energy by pumping an ion up its concentration gradient. This potential energy could then be used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis.
The sodium/phosphate cotransporter is a member of the phosphate:Na+ symporter (PNaS) family within the TOG Superfamily of transport proteins as specified in the Transporter Classification Database (TCDB).

A symporter is an integral membrane protein that is involved in the transport of two different molecules across the cell membrane in the same direction. The symporter works in the plasma membrane and molecules are transported across the cell membrane at the same time, and is, therefore, a type of cotransporter. The transporter is called a symporter, because the molecules will travel in the same direction in relation to each other. This is in contrast to the antiport transporter. Typically, the ion(s) will move down the electrochemical gradient, allowing the other molecule(s) to move against the concentration gradient. The movement of the ion(s) across the membrane is facilitated diffusion, and is coupled with the active transport of the molecule(s). In symport, two molecule move in a 'similar direction' at the 'same time'. For example, the movement of glucose along with sodium ions. It exploits the uphill movement of other molecules from low to high concentration, which is against the electrochemical gradient for the transport of solute molecules downhill from higher to lower concentration.
A neurotransmitter sodium symporter (NSS) (TC# 2.A.22) is type of neurotransmitter transporter that catalyzes the uptake of a variety of neurotransmitters, amino acids, osmolytes and related nitrogenous substances by a solute:Na+ symport mechanism. The NSS family is a member of the APC superfamily. Its constituents have been found in bacteria, archaea and eukaryotes.
GABA transporters are a family of neurotransmitter / sodium symporters, belonging to the solute carrier 6 (SLC6) family. They are found in various regions of the brain in different cell types, such as neurons and astrocytes.

Members of the Solute:Sodium Symporter (SSS) Family (TC# 2.A.21) catalyze solute:Na+ symport. The SSS family is within the APC Superfamily. The solutes transported may be sugars, amino acids, organo cations such as choline, nucleosides, inositols, vitamins, urea or anions, depending on the system. Members of the SSS family have been identified in bacteria, archaea and eukaryotes. Almost all functionally well-characterized members normally catalyze solute uptake via Na+ symport.

Bacterial Leucine Transporter (LeuT) is a bundled twelve alpha helix protein which belongs to the family of transporters that shuttle amino acids in and out of bacterial cells. Specialized in small hydrophobic amino acids such as leucine and alanine, this transporter is powered by the gradient of sodium ions that is normally maintained by healthy cells across their membranes. LeuT acts as a symporter, which means that it links the passage of a sodium ion across the cell membrane with the transport of the amino acid in the same direction. It was first crystallized to understand the inner molecular mechanisms of antidepressant's work since it has a close resemblance with the human neurotransmitter transporters that these drugs block, thus inhibiting the reuptake of chemical messengers across the cell membrane of nerve axons and glial cells.
The amino acid-polyamine-organocation (APC) superfamily is the second largest superfamily of secondary carrier proteins currently known, and it contains several Solute carriers. Originally, the APC superfamily consisted of subfamilies under the transporter classification number. This superfamily has since been expanded to include eighteen different families.
Members of the Alanine or Glycine:Cation Symporter (AGCS) Family (TC# 2.A.25) transport alanine and/or glycine in symport with Na+ and or H+.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.
The Ca2+:cation antiporter (CaCA) family (TC# 2.A.19) is a member of the cation diffusion facilitator (CDF) superfamily. This family should not be confused with the Ca2+:H+ Antiporter-2 (CaCA2) Family (TC# 2.A.106) which belongs to the Lysine Exporter (LysE) Superfamily. Proteins of the CaCA family are found ubiquitously, having been identified in animals, plants, yeast, archaea and divergent bacteria. Members of this family facilitate the antiport of calcium ion with another cation.
Na+/H+ antiporter A (NhaA) family (TC# 2.A.33) contains a number of bacterial sodium-proton antiporter (SPAP) proteins. These are integral membrane proteins that catalyse the exchange of H+ for Na+ in a manner that is highly pH dependent. Homologues have been sequenced from a number of bacteria and archaea. Prokaryotes possess multiple paralogues. A representative list of the proteins that belong to the NhaA family can be found in the Transporter Classification Database.

The Monovalent Cation:Proton Antiporter-1 (CPA1) Family (TC# 2.A.36) is a large family of proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants and animals. The CPA1 family belongs to the VIC superfamily. Transporters from eukaryotes have been functionally characterized to catalyze Na+:H+ exchange. Their primary physiological functions are thought to be in (1) cytoplasmic pH regulation, extruding the H+ generated during metabolism, and (2) salt tolerance (in plants), due to Na+ uptake into vacuoles. Bacterial homologues have also been found to facilitate Na+:H+ antiport, but some also catalyze Li+:H+ antiport or Ca2+:H+ antiport under certain conditions.
The cation:proton antiporter (CPA) superfamily is a superfamily of transport proteins named after one of its constituent members, the monovalent cation:proton antiporter-2 (CPA2).









