 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.054 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
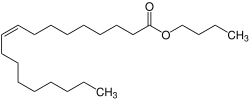
| C22H42O2 | |
| Molar mass | 338.576 g·mol−1 |
| Hazards | |
| GHS labelling: [1] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Butyl oleate is a fatty acid ester and an organic chemical found in liquid form. It has the formula C22H42O2 and the CAS Registry Number 142-77-8. [2] It is REACH registered and produced or imported into the European Union with the EC number of 205-559-6.