
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
Pyruvic acid (IUPAC name: 2-Oxopropanoic acid, also called Acetoic acid or Acetocarboxylic acid) (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.

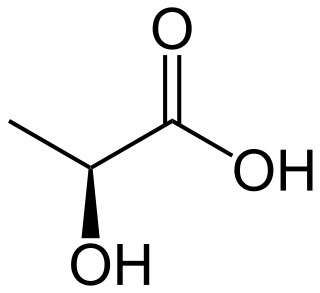
Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate. The name of the derived acyl group is lactoyl.
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.

Lactic acidosis is a medical condition characterized by a build-up of lactate in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates due to a problem with the body's oxidative metabolism.

Anaerobic exercise is a type of exercise that breaks down glucose in the body without using oxygen; anaerobic means "without oxygen". In practical terms, this means that anaerobic exercise is more intense, but shorter in duration than aerobic exercise.

The Cori cycle, named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and converted to glucose, which then returns to the muscles and is cyclically metabolized back to lactate.
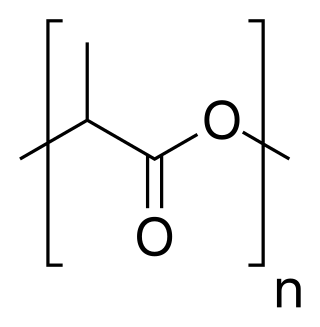
Polylactic acid, also known as poly(lactic acid) poly, lactic, acid or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Calcium lactate is a white crystalline salt with formula C
6H
10CaO
6, consisting of two lactate anions H
3C(CHOH)CO−
2 for each calcium cation Ca2+
. It forms several hydrates, the most common being the pentahydrate C
6H
10CaO
6·5H
2O.
α-Hydroxy acids, or alpha hydroxy acids (AHAs), are a class of chemical compounds that consist of a carboxylic acid with a hydroxyl group substituent on the adjacent (alpha) carbon. Prominent examples are glycolic acid, lactic acid, mandelic acid and citric acid.
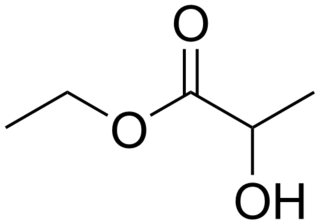
Ethyl lactate, also known as lactic acid ethyl ester, is the organic compound with the formula CH3CH(OH)CO2CH2CH3. It is the ethyl ester of lactic acid. A colorless liquid, it is a chiral ester. Being naturally derived, it is readily available as a single enantiomer. It is commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.

Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.

Mercury(I) sulfate, commonly called mercurous sulphate (UK) or mercurous sulfate (US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.

Sodium lactate is the sodium salt of lactic acid, and has a mild saline taste. It is produced by fermentation of a sugar source, such as corn or beets, and then, by neutralizing the resulting lactic acid to create a compound having the formula NaC3H5O3.

Lithium lactate is a chemical compound, a salt of lithium and lactic acid with the formula CH3CH(OH)COOLi, an amorphous solid, very soluble in water.
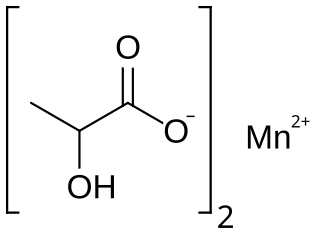
Manganese lactate is an organic chemical compound, a salt of manganese and lactic acid with the formula Mn(C3H5O3)2. The compound forms light pink crystals, soluble in water, forming crystalline hydrates.
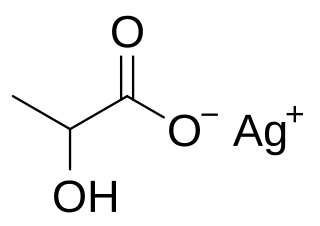
Silver lactate is an organic chemical compound, a salt of silver and lactic acid with the formula CH3CH(OH)COOAg.
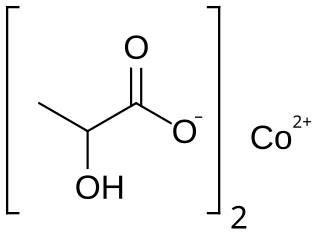
Cobalt lactate is a chemical compound, a salt of cobalt and lactic acid with the formula Co(C3H5O3)2.

Zinc lactate is a chemical compound, a salt of zinc and lactic acid with the formula Zn(C3H5O3)2.

Aluminium lactate is a chemical compound, a salt of aluminium and lactic acid with the formula Al(C3H5O3)3.

















