
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.

Plantago lanceolata is a species of flowering plant in the plantain family Plantaginaceae. It is known by the common names ribwort plantain, narrowleaf plantain, English plantain, ribleaf, lamb's tongue, and buckhorn. It is a common weed on cultivated or disturbed land.
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and Banisteriopsis caapi. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a Reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B. Harmine is also known as banisterin, banisterine, telopathin, telepathine, leucoharmine and yagin, yageine.

Junonia coenia, known as the common buckeye or buckeye, is a butterfly in the family Nymphalidae. Its range covers much of North America and some of Central America, including most of the eastern half of the US, the lower to middle Midwest, the Southwest, southern Canada, and Mexico. Its habitat is open areas with low vegetation and some bare ground. Its original ancestry has been traced to Africa, which then experiences divergence in Asia. The species Junonia grisea, the gray buckeye, is found west of the Rocky Mountains and was formerly a subspecies of Junonia coenia.
Denitrobacterium is a genus of Actinomycetota with a single species, in the family Coriobacteriaceae. Originally isolated from the bovine rumen, Denitrobacterium are non-motile and non-spore forming. The only described species in this genus is Denitrobacterium detoxificans. The specific niche of this bacterium in the bovine rumen is theorized to be the detoxification/metabolism of nitrotoxins and miserotoxin.
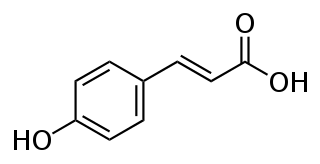
p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

Lamium album, commonly called white dead-nettle, is a flowering plant in the family Lamiaceae. It is native throughout Europe and Asia, growing in a variety of habitats from open grassland to woodland, generally on moist, fertile soils.

Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.

Iridoids are a type of monoterpenoids in the general form of cyclopentanopyran, found in a wide variety of plants and some animals. They are biosynthetically derived from 8-oxogeranial. Iridoids are typically found in plants as glycosides, most often bound to glucose.

Scrophularia californica is a flowering plant in the figwort family which is known by the common names California figwort and California bee plant.

The variable checkerspot or Chalcedon checkerspot is a butterfly in the family Nymphalidae. It is found in western North America, where its range stretches from Alaska in the north to Baja California in the south and extends east through the Rocky Mountains into Colorado, Montana, New Mexico and Wyoming. The butterfly is usually brown or black with extensive white and yellow checkering and some red coloration on the dorsal wing. Adult wingspan is 3.2–5.7 cm (1.3–2.2 in). Adult butterflies feed on nectar from flowers while larvae feed on a variety of plants including snowberry (Symphoricarpos), paintbrush (Castilleja), Buddleja, Diplacus aurantiacus and Scrophularia californica.

Aucubin is an iridoid glycoside. Iridoids are commonly found in plants and function as defensive compounds. Iridoids decrease the growth rates of many generalist herbivores.

Euphydryas cynthia, or Cynthia's fritillary, is a butterfly of the family Nymphalidae. It is found in the Alps and in mountainous areas of Bulgaria in alpine meadows from 400 to 2,300 meters.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 μg/mL, ranging from 3.5 (NEC) to 45 (A549) μg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 synchronized cells at 1-5 μg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.

Euphydryas editha taylori, the Whulge checkerspot or Taylor's checkerspot, is a butterfly native to an area of the northwestern United States and Vancouver Island.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of the pomegranate fruit.

(R)-prunasin is a cyanogenic glycoside related to amygdalin. Chemically, it is the glucoside of (R)-mandelonitrile.
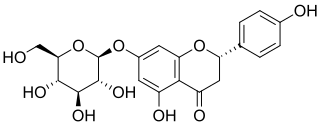
Prunin is a flavanone glycoside found in immature citrus fruits and in tomatoes. Its aglycone form is called naringenin.

Harpagoside is a natural product found in the plant Harpagophytum procumbens, also known as devil's claw. It is the active chemical constituent responsible for the medicinal properties of the plant, which have been used for centuries by the Khoisan people of southern Africa to treat diverse health disorders, including fever, diabetes, hypertension, and various blood related diseases.

Rishitin is a terpenoid compound, produced by some plants belonging to the Solanum family, including the potato and tomato. Rishitin belongs to a heterogeneous group of anti-microbial plant defense compounds termed phytoalexins and is produced upon pathogen attack. Same as the phytoalexin capsidiol, it belongs to the group of sesquiterpenes and is as such an FPP derivative. Rishitin was named after the potato cultivar Rishiri, where it was first discovered in 1968.




















