
A mycorrhiza is a symbiotic association between a fungus and a plant. The term mycorrhiza refers to the role of the fungus in the plant's rhizosphere, its root system. Mycorrhizae play important roles in plant nutrition, soil biology, and soil chemistry.

The Russulaceae are a diverse family of fungi in the order Russulales, with roughly 1,900 known species and a worldwide distribution. They comprise the brittlegills and the milk-caps, well-known mushroom-forming fungi that include some edible species. These gilled mushrooms are characterised by the brittle flesh of their fruitbodies.

An arbuscular mycorrhiza (AM) is a type of mycorrhiza in which the symbiont fungus penetrates the cortical cells of the roots of a vascular plant forming arbuscules. Arbuscular mycorrhiza is a type of endomycorrhiza along with ericoid mycorrhiza and orchid mycorrhiza. They are characterized by the formation of unique tree-like structures, the arbuscules. In addition, globular storage structures called vesicles are often encountered.

Root hair, or absorbent hairs, are outgrowths of epidermal cells, specialized cells at the tip of a plant root. They are lateral extensions of a single cell and are only rarely branched. They are found in the region of maturation, of the root. Root hair cells improve plant water absorption by increasing root surface area to volume ratio which allows the root hair cell to take in more water. The large vacuole inside root hair cells makes this intake much more efficient. Root hairs are also important for nutrient uptake as they are main interface between plants and mycorrhizal fungi.

Laccaria bicolor is a small tan-colored mushroom with lilac gills. It is edible but not choice, and grows in mixed birch and pine woods. It is found in the temperate zones of the globe, in late summer and autumn. L. bicolor is an ectomycorrhizal fungus used as a soil inoculant in agriculture and horticulture.

The ericoid mycorrhiza is a mutualistic relationship formed between members of the plant family Ericaceae and several lineages of mycorrhizal fungi. This symbiosis represents an important adaptation to acidic and nutrient poor soils that species in the Ericaceae typically inhabit, including boreal forests, bogs, and heathlands. Molecular clock estimates suggest that the symbiosis originated approximately 140 million years ago.

Rhizopogon is a genus of ectomycorrhizal basidiomycetes in the family Rhizopogonaceae. Species form hypogeous sporocarps commonly referred to as "false truffles". The general morphological characters of Rhizopogon sporocarps are a simplex or duplex peridium surrounding a loculate gleba that lacks a columnella. Basidiospores are produced upon basidia that are borne within the fungal hymenium that coats the interior surface of gleba locules. The peridium is often adorned with thick mycelial cords, also known as rhizomorphs, that attach the sporocarp to the surrounding substrate. The scientific name Rhizopogon is Greek for 'root' (Rhiz-) 'beard' (-pogon) and this name was given in reference to the rhizomorphs found on sporocarps of many species.
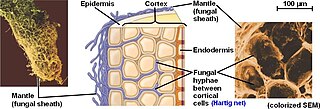
The Hartig net is the network of inward-growing hyphae, that extends into the plant host root, penetrating between plant cells in the root epidermis and cortex in ectomycorrhizal symbiosis. This network is the internal component of fungal morphology in ectomycorrhizal symbiotic structures formed with host plant roots, in addition to a hyphal mantle or sheath on the root surface, and extramatrical mycelium extending from the mantle into the surrounding soil. The Hartig net is the site of mutualistic resource exchange between the fungus and the host plant. Essential nutrients for plant growth are acquired from the soil by exploration and foraging of the extramatrical mycelium, then transported through the hyphal network across the mantle and into the Hartig net, where they are released by the fungi into the root apoplastic space for uptake by the plant. The hyphae in the Hartig net acquire sugars from the plant root, which are transported to the external mycelium to provide a carbon source to sustain fungal growth.

Suillus collinitus is a pored mushroom of the genus Suillus in the family Suillaceae. It is an edible mushroom found in European pine forests. The mushroom has a reddish to chestnut-brown cap that reaches up to 11 cm (4.3 in) in diameter, and a yellow stem measuring up to 7 cm (2.8 in) tall by 1 to 2 cm thick. On the underside of the cap are small angular pores, initially bright yellow before turning greenish-brown with age. A characteristic feature that helps to distinguish it from similar Suillus species, such as S. granulatus, is the pinkish mycelia at the base of the stem.

Tricholoma vaccinum, commonly known as the russet scaly tricholoma, the scaly knight, or the fuzztop, is a fungus of the agaric genus Tricholoma. It produces medium-sized fruit bodies (mushrooms) that have a distinctive hairy reddish-brown cap with a shaggy margin when young. The cap, which can reach a diameter of up to 6.5 cm (2.6 in) wide, breaks up into flattened scales in maturity. It has cream-buff to pinkish gills with brown spots. Its fibrous, hollow stipe is white above and reddish brown below, and measures 4 to 7.5 cm long. Although young fruit bodies have a partial veil, it does not leave a ring on the stipe.
The mycorrhizosphere is the region around a mycorrhizal fungus in which nutrients released from the fungus increase the microbial population and its activities. The roots of most terrestrial plants, including most crop plants and almost all woody plants, are colonized by mycorrhiza-forming symbiotic fungi. In this relationship, the plant roots are infected by a fungus, but the rest of the fungal mycelium continues to grow through the soil, digesting and absorbing nutrients and water and sharing these with its plant host. The fungus in turn benefits by receiving photosynthetic sugars from its host. The mycorrhizosphere consists of roots, hyphae of the directly connected mycorrhizal fungi, associated microorganisms, and the soil in their direct influence.

Mycorrhizal associations have profoundly impacted the evolution of plant life on Earth ever since the initial adaptation of plant life to land. In evolutionary biology, mycorrhizal symbiosis has prompted inquiries into the possibility that symbiosis, not competition, is the main driver of evolution.

Soil carbon storage is an important function of terrestrial ecosystems. Soil contains more carbon than plants and the atmosphere combined. Understanding what maintains the soil carbon pool is important to understand the current distribution of carbon on Earth, and how it will respond to environmental change. While much research has been done on how plants, free-living microbial decomposers, and soil minerals affect this pool of carbon, it is recently coming to light that mycorrhizal fungi—symbiotic fungi that associate with roots of almost all living plants—may play an important role in maintaining this pool as well. Measurements of plant carbon allocation to mycorrhizal fungi have been estimated to be 5 to 20% of total plant carbon uptake, and in some ecosystems the biomass of mycorrhizal fungi can be comparable to the biomass of fine roots. Recent research has shown that mycorrhizal fungi hold 50 to 70 percent of the total carbon stored in leaf litter and soil on forested islands in Sweden. Turnover of mycorrhizal biomass into the soil carbon pool is thought to be rapid and has been shown in some ecosystems to be the dominant pathway by which living carbon enters the soil carbon pool.

An ectomycorrhiza is a form of symbiotic relationship that occurs between a fungal symbiont, or mycobiont, and the roots of various plant species. The mycobiont is often from the phyla Basidiomycota and Ascomycota, and more rarely from the Zygomycota. Ectomycorrhizas form on the roots of around 2% of plant species, usually woody plants, including species from the birch, dipterocarp, myrtle, beech, willow, pine and rose families. Research on ectomycorrhizas is increasingly important in areas such as ecosystem management and restoration, forestry and agriculture.

Ectomycorrhizal extramatrical mycelium is the collection of filamentous fungal hyphae emanating from ectomycorrhizas. It may be composed of fine, hydrophilic hypha which branches frequently to explore and exploit the soil matrix or may aggregate to form rhizomorphs; highly differentiated, hydrophobic, enduring, transport structures.
Dark septate endophytes (DSE) are a group of endophytic fungi characterized by their morphology of melanized, septate, hyphae. This group is likely paraphyletic, and contain conidial as well as sterile fungi that colonize roots intracellularly or intercellularly. Very little is known about the number of fungal taxa within this group, but all are in the Ascomycota. They are found in over 600 plant species and across 114 families of angiosperms and gymnosperms and co-occur with other types of mycorrhizal fungi. They have a wide global distribution and can be more abundant in stressed environments. Much of their taxonomy, physiology, and ecology are unknown.
Orchid mycorrhizae are endomycorrhizal fungi which develop symbiotic relationships with the roots and seeds of plants of the family Orchidaceae. Nearly all orchids are myco-heterotrophic at some point in their life cycle. Orchid mycorrhizae are critically important during orchid germination, as an orchid seed has virtually no energy reserve and obtains its carbon from the fungal symbiont.

Mycorrhiza helper bacteria (MHB) are a group of organisms that form symbiotic associations with both ectomycorrhiza and arbuscular mycorrhiza. MHBs are diverse and belong to a wide variety of bacterial phyla including both Gram-negative and Gram-positive bacteria. Some of the most common MHBs observed in studies belong to the phylas Pseudomonas and Streptomyces. MHBs have been seen to have extremely specific interactions with their fungal hosts at times, but this specificity is lost with plants. MHBs enhance mycorrhizal function, growth, nutrient uptake to the fungus and plant, improve soil conductance, aid against certain pathogens, and help promote defense mechanisms. These bacteria are naturally present in the soil, and form these complex interactions with fungi as plant root development starts to take shape. The mechanisms through which these interactions take shape are not well-understood and needs further study.

Rhizopogon salebrosus is a mushroom species within the Rhizopogon subgenus Amylopogon. R.salebrosus is a monotropoid mycorrhiza that is of vital importance to the ecology of conifer forests, especially in the Pacific Northwest region of North America. Although it is native to North America, R. salebrosus has been found in Europe and its range is generally limited to mountainous regions with sufficient precipitation. The mycoheterotrophic plant, Pterospora andromedea is often found in an obligate association with R. salebrosus in western parts of the U.S. Eastern populations of P. andromedea are typically symbiotic with another Rhizopogon sub species, R. kretzerae.

Mucoromycota is a division within the kingdom fungi. It includes a diverse group of various molds, including the common bread molds Mucor and Rhizopus. It is a sister phylum to Dikarya.

















