An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.

Benzalkonium chloride, also known as alkyldimethylbenzylammonium chloride (ADBAC) and by the trade name Zephiran, is a type of cationic surfactant. It is an organic salt classified as a quaternary ammonium compound. ADBACs have three main categories of use: as a biocide, a cationic surfactant, and a phase transfer agent. ADBACs are a mixture of alkylbenzyldimethylammonium chlorides, in which the alkyl group has various even-numbered alkyl chain lengths.
Carbachol, also known as carbamylcholine and sold under the brand name Miostat among others, is a cholinomimetic drug that binds and activates acetylcholine receptors. Thus it is classified as a cholinergic agonist. It is primarily used for various ophthalmic purposes, such as for treating glaucoma, or for use during ophthalmic surgery. It is generally administered as an ophthalmic solution.

Dry eye syndrome, also known as keratoconjunctivitis sicca, is the condition of having dry eyes. Symptoms include dryness in the eye, irritation, redness, discharge, blurred vision, and easily fatigued eyes. Symptoms range from mild and occasional to severe and continuous. Dry eye syndrome can lead to blurred vision, instability of the tear film, increased risk of damage to the ocular surface such as scarring of the cornea, and changes in the eye including the neurosensory system.
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a tooth. The word topical derives from Greek τοπικόςtopikos, "of a place".
A fabric softener or fabric conditioner is a conditioner that is applied to laundry after it has been washed in a washing machine. A similar, more dilute preparation meant to be applied to dry fabric is known as a wrinkle releaser.

Hydroxypropyl cellulose (HPC) is a derivative of cellulose with both water solubility and organic solubility. It is used as an excipient, and topical ophthalmic protectant and lubricant.

A cream is a preparation usually for application to the skin. Creams for application to mucous membranes such as those of the rectum or vagina are also used. Creams may be considered pharmaceutical products as even cosmetic creams are based on techniques developed by pharmacy and unmedicated creams are highly used in a variety of skin conditions (dermatoses). The use of the finger tip unit concept may be helpful in guiding how much topical cream is required to cover different areas.

Emedastine (trade name Emadine) is a second generation antihistamine used in eye drops to alleviate the symptoms of allergic conjunctivitis. It acts as a H1 receptor antagonist. It works by blocking the action of histamine that causes allergic symptoms. It is used in form of the difumarate. The emedastine difumarate is a white, crystalline, water-soluble fine powder. Emedastine eye drops is usually applied twice a day to the affected eye. When the patients with allergic conjunctivitis were treated with 0.05% emedastine difumarate ophthalmic solution for six weeks, the signs and symptoms such as redness, itching and swelling of the eyes were relieved. Emedastine appears to be devoid of effects on adrenergic, dopaminergic and serotonin receptors. This drug was developed by Alcon, which is global medical company specializing in eye care products.
Kolliphor EL, formerly known as Cremophor EL, is the registered trademark of BASF Corp. for its version of polyethoxylated castor oil. It is prepared by reacting 35 moles of ethylene oxide with each mole of castor oil. The resulting product is a mixture : the major component is the material in which the hydroxyl groups of the castor oil triglyceride have ethoxylated with ethylene oxide to form polyethylene glycol ethers. Minor components are the polyethyelene glycol esters of ricinoleic acid, polyethyelene glycols and polyethyelene glycol ethers of glycerol. Kolliphor EL is a synthetic, nonionic surfactant used to stabilize emulsions of nonpolar materials in water.
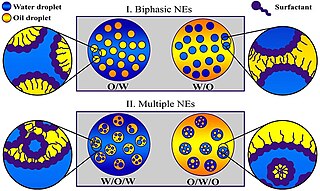
A miniemulsion is a particular type of emulsion. A miniemulsion is obtained by shearing a mixture comprising two immiscible liquid phases, one or more surfactants and, possibly, one or more co-surfactants. They usually have nanodroplets with uniform size distribution and are also known as sub-micron, mini-, and ultra-fine grain emulsions.
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
Thiolated polymers – designated thiomers – are functional polymers used in biotechnology product development with the intention to prolong mucosal drug residence time and to enhance absorption of drugs. The name thiomer was coined by Andreas Bernkop-Schnürch in 2000. Thiomers have thiol bearing side chains. Sulfhydryl ligands of low molecular mass are covalently bound to a polymeric backbone consisting of mainly biodegradable polymers, such as chitosan, hyaluronic acid, cellulose derivatives, pullulan, starch, gelatin, polyacrylates, cyclodextrins, or silicones.

Lipid nanoparticles (LNPs) are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle.

Meibomian gland dysfunction is a chronic disease of the meibomian glands, which is commonly characterized by obstruction of the end of the duct that delivers the secretion produced by the glands to the eye surface, which prevents the glandular secretion from reaching the ocular surface. The dysfunction could be that the amount of secretion produced may be abnormal. Dysfunction could also be related to the quality of the meibum produced. MGD may result in evaporative dry eye, blepharitis, chalazion, unsealed lid during sleep, and meibomian gland atrophy.
Magnesium oil (also referred to as transdermal magnesium, magnesium hexahydrate) is a compound of magnesium chloride dissolved in six molecules of water, with magnesium as the alkaline earth metal and chlorine as the nonmetal. In reality, it is not a "true" oil, as it is not composed of one or more hydrocarbons. Magnesium oil is actually magnesium chloride hexahydrate MgCl2·6H2O. Magnesium oil can be applied to the skin as an alternative to taking a magnesium supplement by mouth, and it is claimed to have health benefits, such as for the treatment of magnesium deficiency, to relieve muscle pain and ache (especially headaches), and to enhance relaxation. However, such use has been described as "scientifically unsupported" due to lack of any convincing data that magnesium is absorbed in significant amounts through the skin. It can also be found as a spray for the mentioned purposes. Magnesium is used in over 600 cellular reactions within the human body, including the immune system. Magnesium oil, with a chemical formula of MgCl2·6H2O has a formula mass of 203.30 g/mol.

Ophthalmic drug administration is the administration of a drug to the eyes, most typically as an eye drop formulation. Topical formulations are used to combat a multitude of diseased states of the eye. These states may include bacterial infections, eye injury, glaucoma, and dry eye. However, there are many challenges associated with topical delivery of drugs to the cornea of the eye.

Topical cream formulation is an emulsion semisolid dosage form that is used for skin external application. Most of the topical cream formulations contain more than 20 per cent of water and volatiles and/or less than 50 per cent of hydrocarbons, waxes, or polyethylene glycols as the vehicle for external skin application. In a topical cream formulation, ingredients are dissolved or dispersed in either a water-in-oil (W/O) emulsion or an oil-in-water (O/W) emulsion. The topical cream formulation has a higher content of oily substance than gel, but a lower content of oily ingredient than ointment. Therefore, the viscosity of topical cream formulation lies between gel and ointment. The pharmacological effect of the topical cream formulation is confined to the skin surface or within the skin. Topical cream formulation penetrates through the skin by transcellular route, intercellular route, or trans-appendageal route. Topical cream formulation is used for a wide range of diseases and conditions, including atopic dermatitis (eczema), psoriasis, skin infection, acne, and wart. Excipients found in a topical cream formulation include thickeners, emulsifying agents, preservatives, antioxidants, and buffer agents. Steps required to manufacture a topical cream formulation include excipient dissolution, phase mixing, introduction of active substances, and homogenization of the product mixture.
Penetration enhancers are chemical compounds that can facilitate the penetration of active pharmaceutical ingredients (API) into or through the poorly permeable biological membranes. These compounds are used in some pharmaceutical formulations to enhance the penetration of APIs in transdermal drug delivery and transmucosal drug delivery. They typically penetrate into the biological membranes and reversibly decrease their barrier properties.
Topical gels are a topical drug delivery dosage form commonly used in cosmetics and treatments for skin diseases because of their advantages over cream and ointment. They are formed from a mixture of gelator, solvent, active drug, and other excipients, and can be classified into organogels and hydrogels. Drug formulation and preparation methods depend on the properties of the gelators, solvents, drug and excipients used.














