
Ants are eusocial insects of the family Formicidae and, along with the related wasps and bees, belong to the order Hymenoptera. Ants evolved from vespoid wasp ancestors in the Cretaceous period. More than 13,800 of an estimated total of 22,000 species have been classified. They are easily identified by their geniculate (elbowed) antennae and the distinctive node-like structure that forms their slender waists.
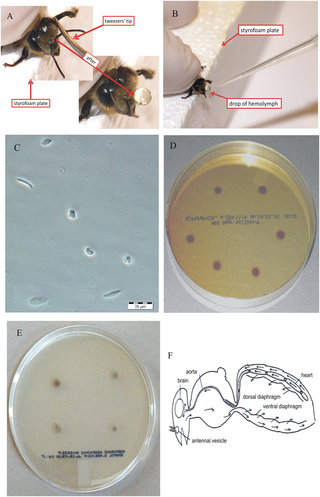
Hemolymph, or haemolymph, is a fluid, analogous to the blood in vertebrates, that circulates in the interior of the arthropod (invertebrate) body, remaining in direct contact with the animal's tissues. It is composed of a fluid plasma in which hemolymph cells called hemocytes are suspended. In addition to hemocytes, the plasma also contains many chemicals. It is the major tissue type of the open circulatory system characteristic of arthropods. In addition, some non-arthropods such as mollusks possess a hemolymphatic circulatory system.

Honeypot ants, also called honey ants, are ants which have specialized workers that consume large amounts of food to the point that their abdomens swell enormously. Other ants then extract nourishment from them, through the process of trophallaxis. They function as living larders. Honeypot ants belong to any of several genera, including Myrmecocystus and Camponotus. They were first documented in 1881 by Henry C. McCook, and described further in 1908 by William Morton Wheeler.

The Formicinae are a subfamily within the Formicidae containing ants of moderate evolutionary development.

Weaver ants or green ants are eusocial insects of the Hymenoptera family Formicidae belonging to the tribe Oecophyllini. Weaver ants live in trees and are known for their unique nest building behaviour where workers construct nests by weaving together leaves using larval silk. Colonies can be extremely large consisting of more than a hundred nests spanning numerous trees and containing more than half a million workers. Like many other ant species, weaver ants prey on small insects and supplement their diet with carbohydrate-rich honeydew excreted by scale insects (Hemiptera). Weaver ant workers exhibit a clear bimodal size distribution, with almost no overlap between the size of the minor and major workers. The major workers are approximately 8–10 mm (0.31–0.39 in) in length and the minors approximately half the length of the majors. Major workers forage, defend, maintain, and expand the colony whereas minor workers tend to stay within the nests where they care for the brood and 'milk' scale insects in or close to the nests.

Carpenter ants are large ants indigenous to many forested parts of the world.
Polyrhachis sokolova is a species of ant from Australia and New Guinea that recently was discovered to be capable of surviving tidal inundations.

The banded sugar ant, also known as the sugar ant, is a species of ant native to Australia. A member of the genus Camponotus in the subfamily Formicinae, it was described by German entomologist Wilhelm Ferdinand Erichson in 1842. Its common name refers to the ant's liking for sugar and sweet food, as well as the distinctive orange-brown band that wraps around its gaster.

Iridomyrmex is a genus of ants called rainbow ants first described by Austrian entomologist Gustav Mayr in 1862. He placed the genus in the subfamily Dolichoderinae of the family Formicidae. It has 79 described species and five fossil species. Most of these ants are native to Australia; others are found in Asia and Oceania, and they have been introduced to Brazil, New Zealand, and the United Arab Emirates. Fossil species are known from China, France, and the United States.

The meat ant, also known as the gravel ant or southern meat ant, is a species of ant endemic to Australia. A member of the genus Iridomyrmex in the subfamily Dolichoderinae, it was described by British entomologist Frederick Smith in 1858. The meat ant is associated with many common names due to its appearance, nest-building behaviour and abundance, of which its specific name, purpureus, refers to its coloured appearance. It is among the best-known species of ant found throughout Australia; it occurs in almost all states and territories except for Tasmania. Its enormous distribution, aggression and ecological importance have made this ant a dominant species.
Phragmosis is any method by which an animal defends itself in its burrow, by using its own body as a barrier. This term was originally coined by W.M. Wheeler (1927), while describing the defensive technique exhibited by insects. Wheeler observed the positioning of specially modified body structures to block nest entrances, as exhibited in various insect species. The term phragmosis has since been further extended beyond just insects.

Amoimyrmex striatus is a species of leafcutter ant found in the Neotropics.

Polyrhachis is a genus of formicine ants found in the Old World with over 600 species. The genus is yet to be comprehensively resolved and contains many varied species including nest-weavers, swimming workers, soil and tree-dwellers. The first fossil record of this genus was of Polyrhachis annosa from the Miocene.

Daceton armigerum is a Neotropical species of arboreal ants, distributed throughout northern South America. D. armigerum combines several traits generally noted in some other arboreal ants i.e., populous colonies, large and/or polydomous nests, intra- and interspecific aggressiveness, trophobiosis, and capturing prey by spread-eagling them.

Camponotus floridanus, or Florida carpenter ant, is a species of ant in the genus Camponotus. First described as Formica floridana by Buckley in 1866, the species was moved to Camponotus by Mayr in 1886. The ant is widespread in Florida and occurs as far north as North Carolina and as far west as Mississippi.

The black-headed sugar ant, also known as the brown sugar ant, is a species of Formicinae ant endemic to Australia. Found throughout most states, the species is a member of the genus Camponotus, a cosmopolitan genus of ants commonly known as carpenter ants. It was formally described and named by British entomologist Frederick Smith in 1858. These ants are characterised by their black head, reddish-brown mesosoma and black gaster, which can change in colour.
Camponotus textor, also known as Brazilian weaver ant, is a species of fairly common tree-dwelling ant native to South and Central America. It is believed to include a number of cryptic species, and previously were considered synonymous to the cavity-dwelling ant Camponotus senex, now thought to be only distantly-related.

Colobopsis is a genus of ant in the subfamily Formicinae. This genus was first described in 1861 by Mayr and contains 95 species. The type species is Colobopsis truncata.

Camponotus ligniperda, the brown-black carpenter ant, is a common species of carpenter ant distributed widely throughout Europe. Found in a variety of woodland habitats, they commonly nest on the ground in dry tree stumps, dead fallen trees, or beneath stones and wooden logs that are partially buried. C. ligniperda is an ecologically dominant species wherever it is found due to both its large size and particularly aggressive nature.














